ICOS maintains the T follicular helper cell phenotype by down-regulating Krüppel-like factor 2
- PMID: 25646266
- PMCID: PMC4322049
- DOI: 10.1084/jem.20141432
ICOS maintains the T follicular helper cell phenotype by down-regulating Krüppel-like factor 2
Abstract
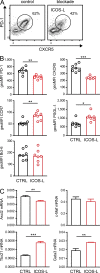
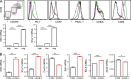
The co-stimulators ICOS (inducible T cell co-stimulator) and CD28 are both important for T follicular helper (TFH) cells, yet their individual contributions are unclear. Here, we show that each molecule plays an exclusive role at different stages of TFH cell development. While CD28 regulated early expression of the master transcription factor Bcl-6, ICOS co-stimulation was essential to maintain the phenotype by regulating the novel TFH transcription factor Klf2 via Foxo1. Klf2 directly binds to Cxcr5, Ccr7, Psgl-1, and S1pr1, and low levels of Klf2 were essential to maintain this typical TFH homing receptor pattern. Blocking ICOS resulted in relocation of fully developed TFH cells back to the T cell zone and reversion of their phenotype to non-TFH effector cells, which ultimately resulted in breakdown of the germinal center response. Our study describes for the first time the exclusive role of ICOS and its downstream signaling in the maintenance of TFH cells by controlling their anatomical localization in the B cell follicle.
© 2015 Weber et al.
Figures








References
-
- Bauquet A.T., Jin H., Paterson A.M., Mitsdoerffer M., Ho I.C., Sharpe A.H., and Kuchroo V.K.. 2009. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 10:167–175 10.1038/ni.1690 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

