The centrosome and its duplication cycle
- PMID: 25646378
- PMCID: PMC4315929
- DOI: 10.1101/cshperspect.a015800
The centrosome and its duplication cycle
Abstract
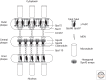
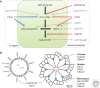
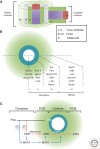
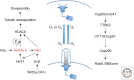
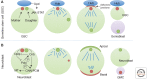
The centrosome was discovered in the late 19th century when mitosis was first described. Long recognized as a key organelle of the spindle pole, its core component, the centriole, was realized more than 50 or so years later also to comprise the basal body of the cilium. Here, we chart the more recent acquisition of a molecular understanding of centrosome structure and function. The strategies for gaining such knowledge were quickly developed in the yeasts to decipher the structure and function of their distinctive spindle pole bodies. Only within the past decade have studies with model eukaryotes and cultured cells brought a similar degree of sophistication to our understanding of the centrosome duplication cycle and the multiple roles of this organelle and its component parts in cell division and signaling. Now as we begin to understand these functions in the context of development, the way is being opened up for studies of the roles of centrosomes in human disease.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



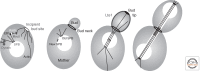
References
-
- Abrieu A, Brassac T, Galas S, Fisher D, Labbe JC, Doree M 1998. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci 111: 1751–1757. - PubMed
-
- Adams IR, Kilmartin JV 2000. Spindle pole body duplication: A model for centrosome duplication? Trend Cell Biol 10: 329–335. - PubMed
-
- Alfa CE, Ducommun B, Beach D, Hyams JS 1990. Distinct nuclear and spindle pole body populations of cyclin-cdc2 in fission yeast. Nature 347: 680–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
