Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer's pathologies
- PMID: 25646590
- PMCID: PMC4445743
- DOI: 10.1038/tp.2014.138
Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer's pathologies
Abstract
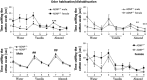
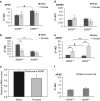
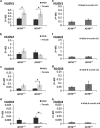
Activity-dependent neuroprotective protein (ADNP) is a most frequent autism spectrum disorder (ASD)-associated gene and the only protein significantly decreasing in the serum of Alzheimer's disease (AD) patients. Is ADNP associated with ASD being more prevalent in boys and AD more prevalent in women? Our results revealed sex-related learning/memory differences in mice, reflecting hippocampal expression changes in ADNP and ADNP-controlled AD/ASD risk genes. Hippocampal ADNP transcript content was doubled in male vs female mice, with females showing equal expression to ADNP haploinsufficient (ADNP(+/)(-)) males and no significant genotype-associated reduction. Increased male ADNP expression was replicated in human postmortem hippocampal samples. The hippocampal transcript for apolipoprotein E (the major risk gene for AD) was doubled in female mice compared with males, and further doubled in the ADNP(+/-) females, contrasting a decrease in ADNP(+/-) males. Previously, overexpression of the eukaryotic translation initiation factor 4E (eIF4E) led to ASD-like phenotype in mice. Here, we identified binding sites on ADNP for eIF4E and co-immunoprecipitation. Furthermore, hippocampal eIF4E expression was specifically increased in young ADNP(+/-) male mice. Behaviorally, ADNP(+/-) male mice exhibited deficiencies in object recognition and social memory compared with ADNP(+/+) mice, while ADNP(+/-) females were partially spared. Contrasting males, which preferred novel over familiar mice, ADNP(+/+) females showed no preference to novel mice and ADNP(+/-) females did not prefer mice over object. ADNP expression, positioned as a master regulator of key ASD and AD risk genes, introduces a novel concept of hippocampal gene-regulated sexual dimorphism and an ADNP(+/-) animal model for translational psychiatry.
Figures


Similar articles
-
Sexual divergence in microtubule function: the novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory.Mol Psychiatry. 2016 Oct;21(10):1467-76. doi: 10.1038/mp.2015.208. Epub 2016 Jan 19. Mol Psychiatry. 2016. PMID: 26782054
-
ADNP is essential for sex-dependent hippocampal neurogenesis, through male unfolded protein response and female mitochondrial gene regulation.Mol Psychiatry. 2025 Jun;30(6):2696-2706. doi: 10.1038/s41380-024-02879-w. Epub 2024 Dec 23. Mol Psychiatry. 2025. PMID: 39715923 Free PMC article.
-
Sexual divergence in activity-dependent neuroprotective protein impacting autism, schizophrenia, and Alzheimer's disease.J Neurosci Res. 2017 Jan 2;95(1-2):652-660. doi: 10.1002/jnr.23808. J Neurosci Res. 2017. PMID: 27870441 Review.
-
Atypical Auditory Brainstem Response and Protein Expression Aberrations Related to ASD and Hearing Loss in the Adnp Haploinsufficient Mouse Brain.Neurochem Res. 2019 Jun;44(6):1494-1507. doi: 10.1007/s11064-019-02723-6. Epub 2019 Jan 18. Neurochem Res. 2019. PMID: 30659505
-
ADNP Plays a Key Role in Autophagy: From Autism to Schizophrenia and Alzheimer's Disease.Bioessays. 2017 Nov;39(11). doi: 10.1002/bies.201700054. Epub 2017 Sep 21. Bioessays. 2017. PMID: 28940660 Review.
Cited by
-
Activity-dependent neuroprotective protein (ADNP): from autism to Alzheimer's disease.Springerplus. 2015 Jun 12;4(Suppl 1):L37. doi: 10.1186/2193-1801-4-S1-L37. eCollection 2015. Springerplus. 2015. PMID: 27386198 Free PMC article. No abstract available.
-
Chromatin remodeler Activity-Dependent Neuroprotective Protein (ADNP) contributes to syndromic autism.Clin Epigenetics. 2023 Mar 21;15(1):45. doi: 10.1186/s13148-023-01450-8. Clin Epigenetics. 2023. PMID: 36945042 Free PMC article. Review.
-
Proteomic phenotype of cerebral organoids derived from autism spectrum disorder patients reveal disrupted energy metabolism, cellular components, and biological processes.Mol Psychiatry. 2022 Sep;27(9):3749-3759. doi: 10.1038/s41380-022-01627-2. Epub 2022 May 26. Mol Psychiatry. 2022. PMID: 35618886
-
The Eight and a Half Year Journey of Undiagnosed AD: Gene Sequencing and Funding of Advanced Genetic Testing Has Led to Hope and New Beginnings.Front Endocrinol (Lausanne). 2017 May 19;8:107. doi: 10.3389/fendo.2017.00107. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28579975 Free PMC article.
-
Sex-and Region-Dependent Expression of the Autism-Linked ADNP Correlates with Social- and Speech-Related Genes in the Canary Brain.J Mol Neurosci. 2020 Nov;70(11):1671-1683. doi: 10.1007/s12031-020-01700-x. Epub 2020 Sep 14. J Mol Neurosci. 2020. PMID: 32926339
References
-
- Gillberg C, Billstedt E. Autism and Asperger syndrome: coexistence with other clinical disorders. Acta Psychiatr Scand. 2000;102:321–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

