FAX1, a novel membrane protein mediating plastid fatty acid export
- PMID: 25646734
- PMCID: PMC4344464
- DOI: 10.1371/journal.pbio.1002053
FAX1, a novel membrane protein mediating plastid fatty acid export
Abstract
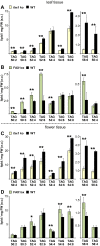
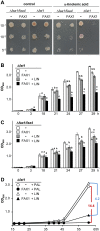
Fatty acid synthesis in plants occurs in plastids, and thus, export for subsequent acyl editing and lipid assembly in the cytosol and endoplasmatic reticulum is required. Yet, the transport mechanism for plastid fatty acids still remains enigmatic. We isolated FAX1 (fatty acid export 1), a novel protein, which inserts into the chloroplast inner envelope by α-helical membrane-spanning domains. Detailed phenotypic and ultrastructural analyses of FAX1 mutants in Arabidopsis thaliana showed that FAX1 function is crucial for biomass production, male fertility and synthesis of fatty acid-derived compounds such as lipids, ketone waxes, or pollen cell wall material. Determination of lipid, fatty acid, and wax contents by mass spectrometry revealed that endoplasmatic reticulum (ER)-derived lipids decreased when FAX1 was missing, but levels of several plastid-produced species increased. FAX1 over-expressing lines showed the opposite behavior, including a pronounced increase of triacyglycerol oils in flowers and leaves. Furthermore, the cuticular layer of stems from fax1 knockout lines was specifically reduced in C29 ketone wax compounds. Differential gene expression in FAX1 mutants as determined by DNA microarray analysis confirmed phenotypes and metabolic imbalances. Since in yeast FAX1 could complement for fatty acid transport, we concluded that FAX1 mediates fatty acid export from plastids. In vertebrates, FAX1 relatives are structurally related, mitochondrial membrane proteins of so-far unknown function. Therefore, this protein family might represent a powerful tool not only to increase lipid/biofuel production in plants but also to explore novel transport systems involved in vertebrate fatty acid and lipid metabolism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Comment in
-
Flagging the fatty acid ferryman.PLoS Biol. 2015 Feb 3;13(2):e1002054. doi: 10.1371/journal.pbio.1002054. eCollection 2015 Feb. PLoS Biol. 2015. PMID: 25646782 Free PMC article.
Similar articles
-
Arabidopsis FAX1 mediated fatty acid export is required for the transcriptional regulation of anther development and pollen wall formation.Plant Mol Biol. 2020 Sep;104(1-2):187-201. doi: 10.1007/s11103-020-01036-5. Epub 2020 Jul 17. Plant Mol Biol. 2020. PMID: 32681357
-
Two activities of long-chain acyl-coenzyme A synthetase are involved in lipid trafficking between the endoplasmic reticulum and the plastid in Arabidopsis.Plant Physiol. 2015 Feb;167(2):351-66. doi: 10.1104/pp.114.250365. Epub 2014 Dec 24. Plant Physiol. 2015. PMID: 25540329 Free PMC article.
-
FAX2 Mediates Fatty Acid Export from Plastids in Developing Arabidopsis Seeds.Plant Cell Physiol. 2019 Oct 1;60(10):2231-2242. doi: 10.1093/pcp/pcz117. Plant Cell Physiol. 2019. PMID: 31198959
-
A role for lipid trafficking in chloroplast biogenesis.Prog Lipid Res. 2008 Sep;47(5):381-9. doi: 10.1016/j.plipres.2008.04.001. Epub 2008 Apr 7. Prog Lipid Res. 2008. PMID: 18440317 Review.
-
Arabidopsis cuticular waxes: advances in synthesis, export and regulation.Prog Lipid Res. 2013 Jan;52(1):110-29. doi: 10.1016/j.plipres.2012.10.002. Epub 2012 Oct 26. Prog Lipid Res. 2013. PMID: 23103356 Review.
Cited by
-
In silico Analysis of Acyl-CoA-Binding Protein Expression in Soybean.Front Plant Sci. 2021 Apr 15;12:646938. doi: 10.3389/fpls.2021.646938. eCollection 2021. Front Plant Sci. 2021. PMID: 33936134 Free PMC article.
-
The roles of chloroplast membrane lipids in abiotic stress responses.Plant Signal Behav. 2020 Nov 1;15(11):1807152. doi: 10.1080/15592324.2020.1807152. Epub 2020 Aug 20. Plant Signal Behav. 2020. PMID: 32815751 Free PMC article.
-
Phosphatidylglycerol Composition Is Central to Chilling Damage in the Arabidopsis fab1 Mutant.Plant Physiol. 2020 Dec;184(4):1717-1730. doi: 10.1104/pp.20.01219. Epub 2020 Oct 7. Plant Physiol. 2020. PMID: 33028639 Free PMC article.
-
Genomic insights into the genetic signatures of selection and seed trait loci in cultivated peanut.J Adv Res. 2022 Dec;42:237-248. doi: 10.1016/j.jare.2022.01.016. Epub 2022 Feb 1. J Adv Res. 2022. PMID: 36513415 Free PMC article.
-
Comprehensive evaluation of fuel properties and complex regulation of intracellular transporters for high oil production in developing seeds of Prunus sibirica for woody biodiesel.Biotechnol Biofuels. 2019 Jan 4;12:6. doi: 10.1186/s13068-018-1347-x. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 30622648 Free PMC article.
References
-
- Koo AJK, Ohlrogge JB, Pollard M (2004) On the export of fatty acids from the chloroplast. J Biol Chem 279: 16101–16110. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

