Effects of enzyme replacement therapy started late in a murine model of mucopolysaccharidosis type I
- PMID: 25646802
- PMCID: PMC4315431
- DOI: 10.1371/journal.pone.0117271
Effects of enzyme replacement therapy started late in a murine model of mucopolysaccharidosis type I
Abstract
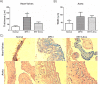
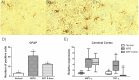
Mucopolysaccharidosis type I (MPS I) is a progressive disorder caused by deficiency of α-L-iduronidase (IDUA), which leads to storage of heparan and dermatan sulphate. It is suggested that early enzyme replacement therapy (ERT) leads to better outcomes, although many patients are diagnosed late and don't receive immediate treatment. This study aims to evaluate the effects of late onset ERT in a MPS I murine model. MPS I mice received treatment from 6 to 8 months of age (ERT 6-8mo) with 1.2mg laronidase/kg every 2 weeks and were compared to 8 months-old wild-type (Normal) and untreated animals (MPS I). ERT was effective in reducing urinary and visceral GAG to normal levels. Heart GAG levels and left ventricular (LV) shortening fraction were normalized but cardiac function was not completely improved. While no significant improvements were found on aortic wall width, treatment was able to significantly reduce heart valves thickening. High variability was found in behavior tests, with treated animals presenting intermediate results between normal and affected mice, without correlation with cerebral cortex GAG levels. Cathepsin D activity in cerebral cortex also did not correlate with behavior heterogeneity. All treated animals developed anti-laronidase antibodies but no correlation was found with any parameters analyzed. However, intermediary results from locomotion parameters analyzed are in accordance with intermediary levels of heart function, cathepsin D, activated glia and reduction of TNF-α expression in the cerebral cortex. In conclusion, even if started late, ERT can have beneficial effects on many aspects of the disease and should be considered whenever possible.
Conflict of interest statement
Figures



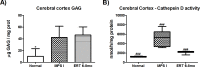
References
-
- Neufeld EF, Muenzer J (2001) The Mucopolysaccharidoses. In: Scriverln C, lnBeaudet A, Sly W, Vaele D, editors. The metabolic and molecular basis of inherited disease. New York: Mc Graw-Hill; pp. 3421–3452. 10.1036/ommbid.165. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

