Chlamydial lung infection induces transient IL-9 production which is redundant for host defense against primary infection
- PMID: 25646821
- PMCID: PMC4315580
- DOI: 10.1371/journal.pone.0115195
Chlamydial lung infection induces transient IL-9 production which is redundant for host defense against primary infection
Abstract
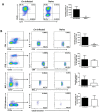
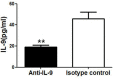
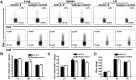
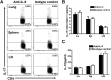
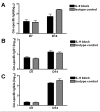
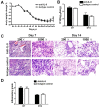
IL-9/Th9 responses are recently found to be important for innate and adaptive immunity particularly in parasitic infections. To date, the study on the role of IL-9 in bacterial infections is limited and the reported data are contradictory. One reported function of IL-9/Th9 is to modulate Th1/Th17 responses. Since our and others' previous work has shown a critical role of Th1 and Th17 cells in host defense against chlamydial lung infection, we here examined the role of IL-9 responses in Chlamydia muridarum (Cm) lung infection, particularly its effect on Th1 and Th17 responses and outcome infection. Our data showed quick but transient IL-9 production in the lung following infection, peaking at day 3 and back to baseline around day 7. CD4+ T cell was the major source of IL-9 production in the lung infection. Blockade of endogenous IL-9 using neutralizing antibody failed to change Interferon-γ (IFN-γ) and IL-17 production by cultured spleen mononuclear cells isolated from Cm infected mice. Similarly, in vivo neutralization of IL-9 failed to show significant effect on T cell (Th1 and Th17) and antibody responses (IgA, IgG1 and IgG2a). Consistently, the neutralization of IL-9 had no significant effect on disease process, including body weight change, bacterial burden and histopathological score. The data suggest that IL-9 production following chlamydial lung infection is redundant for host defense against the intracellular bacteria.
Conflict of interest statement
Figures
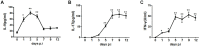

References
-
- Demoulin JB, Renauld JC (1998) Interleukin 9 and its receptor: an overview of structure and function. Int Rev Immunol 16: 345–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

