Endogenous cholinergic neurotransmission contributes to behavioral sensitization to morphine
- PMID: 25647082
- PMCID: PMC4315441
- DOI: 10.1371/journal.pone.0117601
Endogenous cholinergic neurotransmission contributes to behavioral sensitization to morphine
Abstract
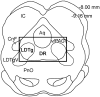
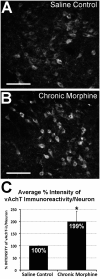
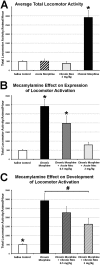
Neuroplasticity in the mesolimbic dopaminergic system is critical for behavioral adaptations associated with opioid reward and addiction. These processes may be influenced by cholinergic transmission arising from the laterodorsal tegmental nucleus (LDTg), a main source of acetylcholine to mesolimbic dopaminergic neurons. To examine this possibility we asked if chronic systemic morphine administration affects expression of genes in ventral and ventrolateral periaqueductal gray at the level of the LDTg using rtPCR. Specifically, we examined gene expression changes in the area of interest using Neurotransmitters and Receptors PCR array between chronic morphine and saline control groups. Analysis suggested that chronic morphine administration led to changes in expression of genes associated, in part, with cholinergic neurotransmission. Furthermore, using a quantitative immunofluorescent technique, we found that chronic morphine treatment produced a significant increase in immunolabeling of the cholinergic marker (vesicular acetylcholine transporter) in neurons of the LDTg. Finally, systemic administration of the nonselective and noncompetitive neuronal nicotinic antagonist mecamylamine (0.5 or 2 mg/kg) dose-dependently blocked the expression, and to a lesser extent the development, of locomotor sensitization. The same treatment had no effect on acute morphine antinociception, antinociceptive tolerance or dependence to chronic morphine. Taken together, the results suggest that endogenous nicotinic cholinergic neurotransmission selectively contributes to behavioral sensitization to morphine and this process may, in part, involve cholinergic neurons within the LDTg.
Conflict of interest statement
Figures


References
-
- Cicero TJ, Inciardi JA, Munoz A (2005) Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. J Pain 6: 662–672. - PubMed
-
- Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5: 483–494. - PubMed
-
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC (2005) Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol 526: 65–76. - PubMed
-
- Pierce RC, Kumaresan V (2006) The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30: 215–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

