Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial
- PMID: 25647203
- PMCID: PMC4374744
- DOI: 10.1001/jama.2015.12
Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial
Abstract
Importance: Severely injured patients experiencing hemorrhagic shock often require massive transfusion. Earlier transfusion with higher blood product ratios (plasma, platelets, and red blood cells), defined as damage control resuscitation, has been associated with improved outcomes; however, there have been no large multicenter clinical trials.
Objective: To determine the effectiveness and safety of transfusing patients with severe trauma and major bleeding using plasma, platelets, and red blood cells in a 1:1:1 ratio compared with a 1:1:2 ratio.
Design, setting, and participants: Pragmatic, phase 3, multisite, randomized clinical trial of 680 severely injured patients who arrived at 1 of 12 level I trauma centers in North America directly from the scene and were predicted to require massive transfusion between August 2012 and December 2013.
Interventions: Blood product ratios of 1:1:1 (338 patients) vs 1:1:2 (342 patients) during active resuscitation in addition to all local standard-of-care interventions (uncontrolled).
Main outcomes and measures: Primary outcomes were 24-hour and 30-day all-cause mortality. Prespecified ancillary outcomes included time to hemostasis, blood product volumes transfused, complications, incidence of surgical procedures, and functional status.
Results: No significant differences were detected in mortality at 24 hours (12.7% in 1:1:1 group vs 17.0% in 1:1:2 group; difference, -4.2% [95% CI, -9.6% to 1.1%]; P = .12) or at 30 days (22.4% vs 26.1%, respectively; difference, -3.7% [95% CI, -10.2% to 2.7%]; P = .26). Exsanguination, which was the predominant cause of death within the first 24 hours, was significantly decreased in the 1:1:1 group (9.2% vs 14.6% in 1:1:2 group; difference, -5.4% [95% CI, -10.4% to -0.5%]; P = .03). More patients in the 1:1:1 group achieved hemostasis than in the 1:1:2 group (86% vs 78%, respectively; P = .006). Despite the 1:1:1 group receiving more plasma (median of 7 U vs 5 U, P < .001) and platelets (12 U vs 6 U, P < .001) and similar amounts of red blood cells (9 U) over the first 24 hours, no differences between the 2 groups were found for the 23 prespecified complications, including acute respiratory distress syndrome, multiple organ failure, venous thromboembolism, sepsis, and transfusion-related complications.
Conclusions and relevance: Among patients with severe trauma and major bleeding, early administration of plasma, platelets, and red blood cells in a 1:1:1 ratio compared with a 1:1:2 ratio did not result in significant differences in mortality at 24 hours or at 30 days. However, more patients in the 1:1:1 group achieved hemostasis and fewer experienced death due to exsanguination by 24 hours. Even though there was an increased use of plasma and platelets transfused in the 1:1:1 group, no other safety differences were identified between the 2 groups.
Trial registration: clinicaltrials.gov Identifier: NCT01545232.
Conflict of interest statement
Figures

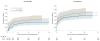
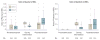

Comment in
-
Mortality and ratio of blood products used in patients with severe trauma.JAMA. 2015 May 26;313(20):2077. doi: 10.1001/jama.2015.4418. JAMA. 2015. PMID: 26010639 No abstract available.
-
Mortality and ratio of blood products used in patients with severe trauma.JAMA. 2015 May 26;313(20):2077-8. doi: 10.1001/jama.2015.4421. JAMA. 2015. PMID: 26010640 No abstract available.
-
Mortality and ratio of blood products used in patients with severe trauma.JAMA. 2015 May 26;313(20):2078. doi: 10.1001/jama.2015.4424. JAMA. 2015. PMID: 26010641 No abstract available.
-
Mortality and ratio of blood products used in patients with severe trauma--reply.JAMA. 2015 May 26;313(20):2078-9. doi: 10.1001/jama.2015.4427. JAMA. 2015. PMID: 26010642 Free PMC article. No abstract available.
-
Comparison of video laryngoscopy versus direct laryngoscopy during urgent endotracheal intubation; trial of the route of early nutritional support in critically ill adults; and transfusion of plasma, platelets, and red blood cells in a 1:1:1 versus a 1:1:2 ratio and mortality in patients with severe trauma.Am J Respir Crit Care Med. 2015 Oct 1;192(7):892-4. doi: 10.1164/rccm.201504-0691RR. Am J Respir Crit Care Med. 2015. PMID: 26275119 No abstract available.
-
Response to: "Misunderstanding the PROPPR trial".Transfusion. 2017 Aug;57(8):2057-2058. doi: 10.1111/trf.14197. Transfusion. 2017. PMID: 28782821 No abstract available.
-
Misunderstanding the PROPPR trial.Transfusion. 2017 Aug;57(8):2056. doi: 10.1111/trf.14200. Transfusion. 2017. PMID: 28782822 No abstract available.
References
-
- US Centers for Disease Control and Preventionl. [Accessed December 21, 2014];Injury prevention and control: data and statistics. 2012 http://webappa.cdc.gov/cgi-bin/broker.exe.
-
- Rhee P, Joseph B, Pandit V, et al. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. - PubMed
-
- Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–310. - PubMed
-
- Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: the surgeon’s perspective. Hematology Am Soc Hematol Educ Program. 2013;2013:656–659. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

