CXCL13 promotes the effect of bone marrow mesenchymal stem cells (MSCs) on tendon-bone healing in rats and in C3HIOT1/2 cells
- PMID: 25647417
- PMCID: PMC4346887
- DOI: 10.3390/ijms16023178
CXCL13 promotes the effect of bone marrow mesenchymal stem cells (MSCs) on tendon-bone healing in rats and in C3HIOT1/2 cells
Abstract
Objectives: Mesenchymal stem cells (MSCs) are potential effective therapy for tissue repair and bone regeneration. In present study, the effects of CXC chemokine ligand-13 (CXCL13) were evaluated on tendon-bone healing of rats.
Methods: Tendon bone healing of the rat model was established and biomechanical testing was performed at 2, 4, 8 weeks after surgery. Murine mesenchymal cell line (C3HIOT1/2 cells) was cultured. The expression of miRNA-23a was detected by real-time PCR. The protein expression of ERK1/2, JNK and p38 was detected by western blotting. MiR-23a mimic and inhibitor were used to overexpress or silence the expression of miR-23a.
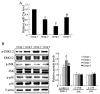
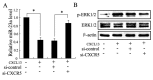
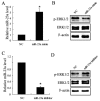
Results: MSCs significantly elevated the levels of ultimate load to failure, stiffness and stress in specimens of rats, the effects of which were enhanced by CXCL13. The expression of miR-23a was down-regulated and the protein of ERK1/2 level was up-regulated by CXCL13 treatment in both in vivo and in vitro experiments. ERK1/2 expression was elevated by overexpression of miR-23a and reduced by miR-23a inhibitor.
Conclusions: These findings revealed that CXCL13 promoted the tendon-bone healing in rats with MSCs treatment, and implied that the activation of ERK1/2 via miR-23a was involved in the process of MSCs treated bone regeneration.
Figures


Similar articles
-
TOB1 Deficiency Enhances the Effect of Bone Marrow-Derived Mesenchymal Stem Cells on Tendon-Bone Healing in a Rat Rotator Cuff Repair Model.Cell Physiol Biochem. 2016;38(1):319-29. doi: 10.1159/000438632. Epub 2016 Jan 27. Cell Physiol Biochem. 2016. PMID: 26824451
-
CXCL13 inhibits microRNA-23a through PI3K/AKT signaling pathway in adipose tissue derived-mesenchymal stem cells.Biomed Pharmacother. 2016 Oct;83:876-880. doi: 10.1016/j.biopha.2016.07.059. Epub 2016 Aug 8. Biomed Pharmacother. 2016. PMID: 27509223
-
High glucose prevents osteogenic differentiation of mesenchymal stem cells via lncRNA AK028326/CXCL13 pathway.Biomed Pharmacother. 2016 Dec;84:544-551. doi: 10.1016/j.biopha.2016.09.058. Epub 2016 Sep 28. Biomed Pharmacother. 2016. PMID: 27693963
-
Directed Differentiation and Paracrine Mechanisms of Mesenchymal Stem Cells: Potential Implications for Tendon Repair and Regeneration.Curr Stem Cell Res Ther. 2017;12(6):447-454. doi: 10.2174/1574888X12666170502102423. Curr Stem Cell Res Ther. 2017. PMID: 28464787 Review.
-
Making Them Commit: Strategies to Influence Phenotypic Differentiation in Mesenchymal Stem Cells.Sports Med Arthrosc Rev. 2018 Jun;26(2):64-69. doi: 10.1097/JSA.0000000000000187. Sports Med Arthrosc Rev. 2018. PMID: 29722765 Review.
Cited by
-
Baicalein Accelerates Tendon-Bone Healing via Activation of Wnt/β-Catenin Signaling Pathway in Rats.Biomed Res Int. 2018 Mar 6;2018:3849760. doi: 10.1155/2018/3849760. eCollection 2018. Biomed Res Int. 2018. PMID: 29693006 Free PMC article.
-
The transition of M-CSF-derived human macrophages to a growth-promoting phenotype.Blood Adv. 2020 Nov 10;4(21):5460-5472. doi: 10.1182/bloodadvances.2020002683. Blood Adv. 2020. PMID: 33166408 Free PMC article.
-
Strategies to promote tendon-bone healing after anterior cruciate ligament reconstruction: Present and future.Front Bioeng Biotechnol. 2023 Mar 13;11:1104214. doi: 10.3389/fbioe.2023.1104214. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36994361 Free PMC article. Review.
-
Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part II: treatment options.J Exp Orthop. 2018 Sep 24;5(1):38. doi: 10.1186/s40634-018-0145-5. J Exp Orthop. 2018. PMID: 30251203 Free PMC article. Review.
-
Strategies for promoting tendon-bone healing: Current status and prospects.Front Bioeng Biotechnol. 2023 Jan 27;11:1118468. doi: 10.3389/fbioe.2023.1118468. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36777256 Free PMC article. Review.
References
-
- Backesjo C.M., Li Y., Lindgren U., Haldosen L.A. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J. Bone Miner. Res. 2006;21:993–1002. - PubMed
-
- Zhou S.B., Wang J., Chiang C.A., Sheng L.L., Li Q.F. Mechanical stretch upregulates SDF-1α in skin tissue and induces migration of circulating bone marrow-derived stem cells into the expanded skin. Stem Cells. 2013;31:2703–2713. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

