Perioperative trajectory of patient reported symptoms: a pilot study in gynecologic oncology patients
- PMID: 25647455
- PMCID: PMC4711289
- DOI: 10.1016/j.ygyno.2015.01.547
Perioperative trajectory of patient reported symptoms: a pilot study in gynecologic oncology patients
Abstract
Objective: With the growing focus on patient-centered care, patient reported outcomes (PROs) are becoming an important component to clinical trials and quality metrics. The objective of this study was to pilot the collection of patient reported symptom burden in women undergoing surgery in a gynecologic oncology practice.
Methods: Perioperative patient reported symptom burden was measured for women undergoing laparotomy on the gynecologic oncology service at the University of Texas MD Anderson Cancer Center. Symptoms were assessed using the M.D. Anderson Symptom Inventory (MDASI-OC), a 27 item tool validated for use in patients with ovarian cancer. The MDASI-OC was administered as a preoperative baseline, daily while admitted to the hospital after surgery, twice a week on the first week after discharge and then weekly until 8 weeks postoperatively.
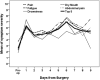
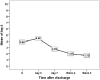
Results: 29 patients were evaluable. Seventy-five percent of patients had a diagnosis of ovarian cancer. Of those patients, half underwent a primary debulking surgery and the other half had neoadjuvant chemotherapy prior to interval cytoreductive surgery. In the postoperative inpatient setting, the five symptoms with the highest overall burden were fatigue, pain, abdominal pain, dry mouth and drowsiness. Longitudinal change of the top 5 symptoms during hospitalization did not show any significant difference between those who had neoadjuvant chemotherapy and those who did not.
Conclusion: The collection of longitudinal PROs to assess symptom burden is feasible in patients undergoing gynecologic oncology surgery. Patient reported outcomes are a crucial component of patient-centered research and the longitudinal collection and analysis of symptom burden can allow for more meaningful comparisons of surgical technique and perioperative care.
Keywords: Patient reported outcomes; Surgery; Symptom burden.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest: The authors have no relevant conflicts of interest to report.
Figures


References
-
- Wu AW, et al. Adding the patient perspective to comparative effectiveness research. Health Aff (Millwood) 2010;29(10):1863–71. - PubMed
-
- Bilimoria KY, Cella D, Butt Z. Current Challenges in Using Patient-Reported Outcomes for Surgical Care and Performance Measurement: Everybody Wants to Hear From the Patient, but Are We Ready to Listen? JAMA Surg. 2014 - PubMed
-
- Basch E, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–55. - PubMed
-
- Joly F, et al. Quality of life and patient-reported outcomes in endometrial cancer clinical trials: a call for action! Int J Gynecol Cancer. 2014;24(9):1693–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

