Dopamine increases CD14+CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis
- PMID: 25647501
- PMCID: PMC4315499
- DOI: 10.1371/journal.pone.0117450
Dopamine increases CD14+CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis
Abstract
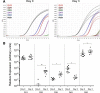
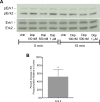
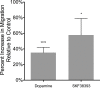
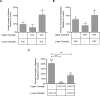
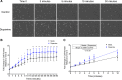
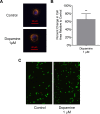
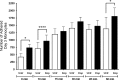
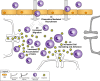
Drug abuse is a major comorbidity of HIV infection and cognitive disorders are often more severe in the drug abusing HIV infected population. CD14+CD16+ monocytes, a mature subpopulation of peripheral blood monocytes, are key mediators of HIV neuropathogenesis. Infected CD14+CD16+ monocyte transmigration across the blood brain barrier mediates HIV entry into the brain and establishes a viral reservoir within the CNS. Despite successful antiretroviral therapy, continued influx of CD14+CD16+ monocytes, both infected and uninfected, contributes to chronic neuroinflammation and the development of HIV associated neurocognitive disorders (HAND). Drug abuse increases extracellular dopamine in the CNS. Once in the brain, CD14+CD16+ monocytes can be exposed to extracellular dopamine due to drug abuse. The direct effects of dopamine on CD14+CD16+ monocytes and their contribution to HIV neuropathogenesis are not known. In this study, we showed that CD14+CD16+ monocytes express mRNA for all five dopamine receptors by qRT-PCR and D1R, D5R and D4R surface protein by flow cytometry. Dopamine and the D1-like dopamine receptor agonist, SKF38393, increased CD14+CD16+ monocyte migration that was characterized as chemokinesis. To determine whether dopamine affected cell motility and adhesion, live cell imaging was used to monitor the accumulation of CD14+CD16+ monocytes on the surface of a tissue culture dish. Dopamine increased the number and the rate at which CD14+CD16+ monocytes in suspension settled to the dish surface. In a spreading assay, dopamine increased the area of CD14+CD16+ monocytes during the early stages of cell adhesion. In addition, adhesion assays showed that the overall total number of adherent CD14+CD16+ monocytes increased in the presence of dopamine. These data suggest that elevated extracellular dopamine in the CNS of HIV infected drug abusers contributes to HIV neuropathogenesis by increasing the accumulation of CD14+CD16+ monocytes in dopamine rich brain regions.
Conflict of interest statement
Figures

References
-
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, et al. (1992) Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 42: 1736–1739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

