The role of Abcb5 alleles in susceptibility to haloperidol-induced toxicity in mice and humans
- PMID: 25647612
- PMCID: PMC4315575
- DOI: 10.1371/journal.pmed.1001782
The role of Abcb5 alleles in susceptibility to haloperidol-induced toxicity in mice and humans
Abstract
Background: We know very little about the genetic factors affecting susceptibility to drug-induced central nervous system (CNS) toxicities, and this has limited our ability to optimally utilize existing drugs or to develop new drugs for CNS disorders. For example, haloperidol is a potent dopamine antagonist that is used to treat psychotic disorders, but 50% of treated patients develop characteristic extrapyramidal symptoms caused by haloperidol-induced toxicity (HIT), which limits its clinical utility. We do not have any information about the genetic factors affecting this drug-induced toxicity. HIT in humans is directly mirrored in a murine genetic model, where inbred mouse strains are differentially susceptible to HIT. Therefore, we genetically analyzed this murine model and performed a translational human genetic association study.
Methods and findings: A whole genome SNP database and computational genetic mapping were used to analyze the murine genetic model of HIT. Guided by the mouse genetic analysis, we demonstrate that genetic variation within an ABC-drug efflux transporter (Abcb5) affected susceptibility to HIT. In situ hybridization results reveal that Abcb5 is expressed in brain capillaries, and by cerebellar Purkinje cells. We also analyzed chromosome substitution strains, imaged haloperidol abundance in brain tissue sections and directly measured haloperidol (and its metabolite) levels in brain, and characterized Abcb5 knockout mice. Our results demonstrate that Abcb5 is part of the blood-brain barrier; it affects susceptibility to HIT by altering the brain concentration of haloperidol. Moreover, a genetic association study in a haloperidol-treated human cohort indicates that human ABCB5 alleles had a time-dependent effect on susceptibility to individual and combined measures of HIT. Abcb5 alleles are pharmacogenetic factors that affect susceptibility to HIT, but it is likely that additional pharmacogenetic susceptibility factors will be discovered.
Conclusions: ABCB5 alleles alter susceptibility to HIT in mouse and humans. This discovery leads to a new model that (at least in part) explains inter-individual differences in susceptibility to a drug-induced CNS toxicity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

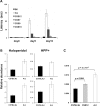
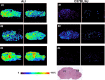

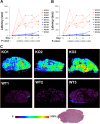


Similar articles
-
A Pharmacogenetic Discovery: Cystamine Protects Against Haloperidol-Induced Toxicity and Ischemic Brain Injury.Genetics. 2016 May;203(1):599-609. doi: 10.1534/genetics.115.184648. Epub 2016 Mar 18. Genetics. 2016. PMID: 26993135 Free PMC article.
-
Genetically determined ABCB5 functionality correlates with pigmentation phenotype and melanoma risk.Biochem Biophys Res Commun. 2013 Jul 5;436(3):536-42. doi: 10.1016/j.bbrc.2013.06.006. Epub 2013 Jun 12. Biochem Biophys Res Commun. 2013. PMID: 23770371 Free PMC article.
-
Drug resistance is conferred on the model yeast Saccharomyces cerevisiae by expression of full-length melanoma-associated human ATP-binding cassette transporter ABCB5.Mol Pharm. 2014 Oct 6;11(10):3452-62. doi: 10.1021/mp500230b. Epub 2014 Aug 22. Mol Pharm. 2014. PMID: 25115303 Free PMC article.
-
Functional significance of genetic polymorphisms in P-glycoprotein (MDR1, ABCB1) and breast cancer resistance protein (BCRP, ABCG2).Drug Metab Pharmacokinet. 2012;27(1):85-105. doi: 10.2133/dmpk.dmpk-11-rv-098. Epub 2011 Nov 29. Drug Metab Pharmacokinet. 2012. PMID: 22123128 Review.
-
Genetic polymorphisms of ATP-binding cassette transporters ABCB1 and ABCC2 and their impact on drug disposition.Curr Drug Targets. 2011 May;12(5):631-46. doi: 10.2174/138945011795378487. Curr Drug Targets. 2011. PMID: 21039333 Review.
Cited by
-
Optimizing a therapy for opiate use disorders: Characterizing ondansetron pharmacokinetics in blood and brain.Clin Transl Sci. 2023 Feb;16(2):216-223. doi: 10.1111/cts.13440. Epub 2022 Nov 9. Clin Transl Sci. 2023. PMID: 36305236 Free PMC article.
-
Twenty-first century mouse genetics is again at an inflection point.Lab Anim (NY). 2025 Jan;54(1):9-15. doi: 10.1038/s41684-024-01491-3. Epub 2024 Nov 26. Lab Anim (NY). 2025. PMID: 39592878 Free PMC article. Review.
-
The Effect of Population Structure on Murine Genome-Wide Association Studies.Front Genet. 2021 Sep 13;12:745361. doi: 10.3389/fgene.2021.745361. eCollection 2021. Front Genet. 2021. PMID: 34589118 Free PMC article.
-
The multiple PDZ domain protein Mpdz/MUPP1 regulates opioid tolerance and opioid-induced hyperalgesia.BMC Genomics. 2016 Apr 29;17:313. doi: 10.1186/s12864-016-2634-1. BMC Genomics. 2016. PMID: 27129385 Free PMC article.
-
Low heritability in pharmacokinetics of talinolol: a pharmacogenetic twin study on the heritability of the pharmacokinetics of talinolol, a putative probe drug of MDR1 and other membrane transporters.Genome Med. 2016 Nov 8;8(1):119. doi: 10.1186/s13073-016-0372-2. Genome Med. 2016. PMID: 27825374 Free PMC article. Clinical Trial.
References
-
- Ohtsuki S, Terasaki T (2007) Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res 24: 1745–1758. - PubMed
-
- Beresford R, Ward A (1987) Haloperidol decanoate. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in psychosis. Drugs 33: 31–49. - PubMed
-
- Parkes GE (1982) Haloperidol: profile of side effects. Johnson DAW, editor. Haloperidol decanoate and the treatment of chronic schizophrenia. New York: ADIS Press; pp. 58–63.
-
- Soares-Weiser K, Fernandez HH (2007) Tardive dyskinesia. Semin Neurol 27: 159–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

