Hypoxia-inducible factor-1α regulates the expression of L-type voltage-dependent Ca(2+) channels in PC12 cells under hypoxia
- PMID: 25648081
- PMCID: PMC4406929
- DOI: 10.1007/s12192-015-0575-2
Hypoxia-inducible factor-1α regulates the expression of L-type voltage-dependent Ca(2+) channels in PC12 cells under hypoxia
Abstract
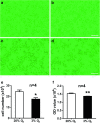
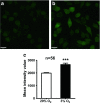
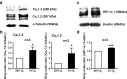
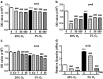
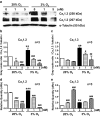
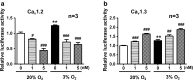
Hypoxia is an important factor in regulation of cell behavior both under physiological and pathological conditions. The mechanisms of hypoxia-induced cell death have not been completely elucidated yet. It is well known that Ca(2+) is critically related to cell survival. Hypoxia-inducible factor-1α (HIF-1α) is a core regulatory factor during hypoxia, and L-type voltage-dependent Ca(2+) channels (L-VDCCs) have been reported to play a critical role in cell survival. This study was conducted to explore the relationship between L-VDCC expression and HIF-1α regulation in PC12 cells under hypoxia. PC12 cells were treated at 20 or 3 % O2 to observe its proliferation and the intracellular calcium concentration. Then, we detected the protein expression of HIF-1α and L-VDCCs subtypes, Cav1.2 and Cav1.3. At last, to verify the relationship between HIF-1α and Cav1.2 and Cav1.3, we got the expression of Cav1.2 and Cav1.3 with Western blot and luciferase report gene assays after PC12 cells were treated by echinomycin, which is an HIF-1α inhibitor. Compared with 20 % O2 (normoxia), 3 % O2 (hypoxia) inhibited cell proliferation, increased the intracellular calcium concentration, and induced protein expression of HIF-1α. The protein expression of two L-VDCCs subtypes expressed in the nervous system, Cav1.2 and Cav1.3, was upregulated by hypoxia and reduced dose dependently by treatment with echinomycin, a HIF-1α inhibitor. Luciferase report gene assays showed that the expression of Cav1.2 and Cav1.3 genes was augmented under 3 % O2. However, echinomycin only slightly and dose dependently decreased expression of the Cav1.2 gene, but not that of the Cav1.3 gene. These data indicated that Cav1.2 might be regulated by HIF-1α as one of its downstream target genes and involved in regulation of PC12 cells death under hypoxia.
Figures
References
-
- Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, Domercq M, Matute C. Amyloid β oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47(3):264–272. doi: 10.1016/j.ceca.2009.12.010. - DOI - PubMed
-
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1α increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27(23):6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

