A Randomized Phase II Trial of Capecitabine Plus Vinorelbine Followed by Docetaxel Versus Adriamycin Plus Cyclophosphamide Followed by Docetaxel as Neoadjuvant Chemotherapy for Breast Cancer
- PMID: 25648092
- PMCID: PMC4506113
- DOI: 10.4143/crt.2014.073
A Randomized Phase II Trial of Capecitabine Plus Vinorelbine Followed by Docetaxel Versus Adriamycin Plus Cyclophosphamide Followed by Docetaxel as Neoadjuvant Chemotherapy for Breast Cancer
Abstract
Purpose: Given the promising activity of capecitabine and vinorelbine in metastatic breast cancer, this randomized phase II trial evaluated the efficacy and safety of this combination as neoadjuvant chemotherapy in breast cancer.
Materials and methods: Patients with operable breast cancer (n=75) were randomly assigned to receive either four cycles of adriamycin 60 mg/m(2) plus cyclophosphamide 600 mg/m(2) every 3 weeks followed by four cycles of docetaxel 75 mg/m(2) every 3 weeks (AC-D) or four cycles of capecitabine 2,000 mg/m(2) (day 1-14) plus vinorelbine 25 mg/m(2) (days 1 and 8) every 3 weeks followed by four cycles of docetaxel 75 mg/m(2) (CV-D). The primary endpoint was pathologic complete response (pCR) in the primary breast (ypT0/is).
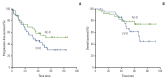
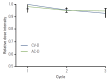
Results: Most patients (84%) had locally advanced (n=41) or inflammatory breast cancer (n=22). pCR rates in the primary breast were 15% (95% confidence interval [CI], 7% to 30%) and 11% (95% CI, 4% to 26%) in the AC-D and CV-D groups, respectively. The overall response rates and 5-year progression-free survival rates in the AC-D and CV-D groups were 62% and 64%, and 51.3% (95% CI, 34.6% to 68.0%) and 30.2% (95% CI, 13.3% to 47.1%), respectively. Although both regimens were well tolerated, CV-D showed less frequent grade 3-4 neutropenia and vomiting than AC-D, whereas manageable diarrhea and hand-foot syndrome were more common in the CV-D group.
Conclusion: CV-D is a feasible and active non-anthracycline-based neoadjuvant chemotherapy regimen for breast cancer.
Keywords: Anthracyclines; Breast neoplasm; Capecitabine; Neoadjuvant therapy; Vinorelbine.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures

References
-
- Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994;73:362–9. - PubMed
-
- Mauriac L, Durand M, Avril A, Dilhuydy JM. Effects of primary chemotherapy in conservative treatment of breast cancer patients with operable tumors larger than 3 cm: results of a randomized trial in a single centre. Ann Oncol. 1991;2:347–54. - PubMed
-
- van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–37. - PubMed
-
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85. - PubMed
-
- Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–13. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

