Establishing conditions for the storage and elution of rabies virus RNA using FTA(®) cards
- PMID: 25648208
- PMCID: PMC4427748
- DOI: 10.1292/jvms.14-0227
Establishing conditions for the storage and elution of rabies virus RNA using FTA(®) cards
Abstract
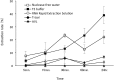
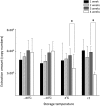
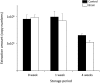
The Flinders Technology Associates filter paper cards (FTA(®) cards) can be used to store nucleic acid from various samples and are easily portable. However, RNA is physicochemically unstable compared with DNA, and appropriate methods have not been established for storage and extraction of RNA from FTA(®) cards. The present study investigated the optimum conditions for storage and elution of viral RNA (vRNA) using rabies virus (RABV) applied to FTA(®) cards. When TE buffer was used, the elution rates of vRNA increased with the length of the elution time. When the cards were stored at -80 °C or -20 °C, vRNA was stable over 3 months. Degradation of vRNAs occurred following storage at 4 °C and room temperature, suggesting that RNA should be extracted from cards as soon as possible if no freezer is available. When we tried to amplify vRNA from RABV-infected animal brains applied to FTA(®) cards and stored at -80 °C for 6 months, we did not detect any amplified products with the primer set for 964 bp of RABV N gene. However, we were able to detect amplified products by increasing the elution time of vRNA from FTA(®) cards from 30 min to 24 hr or by changing the primer sets to amplify 290 bp of N gene. Thus, we recommend extending the elution time for damaged or low concentration samples in FTA(®) cards.
Figures
Similar articles
-
A systematic review of FTA cards® as a tool for viral RNA preservation in fieldwork: Are they safe and effective?Prev Vet Med. 2019 Nov 15;172:104772. doi: 10.1016/j.prevetmed.2019.104772. Epub 2019 Sep 10. Prev Vet Med. 2019. PMID: 31607414 Free PMC article.
-
Use of FTA cards for the storage of breast carcinoma nucleic acid on fine-needle aspiration samples.Cancer Cytopathol. 2015 Oct;123(10):582-92. doi: 10.1002/cncy.21577. Epub 2015 Jun 29. Cancer Cytopathol. 2015. PMID: 26123795
-
Molecular detection and serotyping of infectious bronchitis virus from FTA filter paper.Avian Dis. 2005 Mar;49(1):24-9. doi: 10.1637/7220. Avian Dis. 2005. PMID: 15839408
-
An optimized method for elution of enteroviral RNA from a cellulose-based substrate.J Virol Methods. 2012 Dec;186(1-2):62-7. doi: 10.1016/j.jviromet.2012.08.016. Epub 2012 Sep 6. J Virol Methods. 2012. PMID: 22960199
-
FTA Cards for Preservation of Nucleic Acids for Molecular Assays: A Review on the Use of Cytologic/Tissue Samples.Arch Pathol Lab Med. 2018 Mar;142(3):308-312. doi: 10.5858/arpa.2017-0303-RA. Arch Pathol Lab Med. 2018. PMID: 29494226 Review.
Cited by
-
An inter-laboratory trial as a tool to increase rabies diagnostic capabilities of Sub-Saharan African Veterinary laboratories.PLoS Negl Trop Dis. 2020 Feb 10;14(2):e0008010. doi: 10.1371/journal.pntd.0008010. eCollection 2020 Feb. PLoS Negl Trop Dis. 2020. PMID: 32040472 Free PMC article.
-
A systematic review of FTA cards® as a tool for viral RNA preservation in fieldwork: Are they safe and effective?Prev Vet Med. 2019 Nov 15;172:104772. doi: 10.1016/j.prevetmed.2019.104772. Epub 2019 Sep 10. Prev Vet Med. 2019. PMID: 31607414 Free PMC article.
-
Optimizing Release of Nucleic Acids of African Swine Fever Virus and Influenza A Virus from FTA Cards.Int J Mol Sci. 2021 Nov 29;22(23):12915. doi: 10.3390/ijms222312915. Int J Mol Sci. 2021. PMID: 34884719 Free PMC article.
-
Predominance of low pathogenic avian influenza virus H9N2 in the respiratory co-infections in broilers in Tunisia: a longitudinal field study, 2018-2020.Vet Res. 2023 Oct 3;54(1):88. doi: 10.1186/s13567-023-01204-7. Vet Res. 2023. PMID: 37789451 Free PMC article.
-
Facilitating Cross-border Viral Sequencing Through Nucleic Acid Sample Transport Using Dry Cards.Viruses. 2025 May 31;17(6):804. doi: 10.3390/v17060804. Viruses. 2025. PMID: 40573394 Free PMC article.
References
-
- Linhares D. C., Rovira A., Torremorell M.2012. Evaluation of Flinders Technology Associates cards for collection and transport of samples for detection of Porcine reproductive and respiratory syndrome virus by reverse transcription polymerase chain reaction. J. Vet. Diagn. Invest. 24: 328–332. doi: 10.1177/1040638711429492 - DOI - PubMed
-
- Muthukrishnan M., Singanallur N. B., Ralla K., Villuppanoor S. A.2008. Evaluation of FTA cards as a laboratory and field sampling device for the detection of foot-and-mouth disease virus and serotyping by RT-PCR and real-time RT-PCR. J. Virol. Methods 151: 311–316. doi: 10.1016/j.jviromet.2008.05.020 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

