Haloarchaea and the formation of gas vesicles
- PMID: 25648404
- PMCID: PMC4390858
- DOI: 10.3390/life5010385
Haloarchaea and the formation of gas vesicles
Abstract
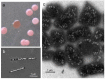
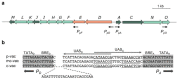
Halophilic Archaea (Haloarchaea) thrive in salterns containing sodium chloride concentrations up to saturation. Many Haloarchaea possess genes encoding gas vesicles, but only a few species, such as Halobacterium salinarum and Haloferax mediterranei, produce these gas-filled, proteinaceous nanocompartments. Gas vesicles increase the buoyancy of cells and enable them to migrate vertically in the water body to regions with optimal conditions. Their synthesis depends on environmental factors, such as light, oxygen supply, temperature and salt concentration. Fourteen gas vesicle protein (gvp) genes are involved in their formation, and regulation of gvp gene expression occurs at the level of transcription, including the two regulatory proteins, GvpD and GvpE, but also at the level of translation. The gas vesicle wall is solely formed of proteins with the two major components, GvpA and GvpC, and seven additional accessory proteins are also involved. Except for GvpI and GvpH, all of these are required to form the gas permeable wall. The applications of gas vesicles include their use as an antigen presenter for viral or pathogen proteins, but also as a stable ultrasonic reporter for biomedical purposes.
Figures



Similar articles
-
Regulation of gas vesicle formation in halophilic archaea.J Mol Microbiol Biotechnol. 2002 May;4(3):175-81. J Mol Microbiol Biotechnol. 2002. PMID: 11931543 Review.
-
Accessory Gvp Proteins Form a Complex During Gas Vesicle Formation of Haloarchaea.Front Microbiol. 2020 Nov 12;11:610179. doi: 10.3389/fmicb.2020.610179. eCollection 2020. Front Microbiol. 2020. PMID: 33281806 Free PMC article.
-
A p-loop motif and two basic regions in the regulatory protein GvpD are important for the repression of gas vesicle formation in the archaeon Haloferax mediterranei.Microbiology (Reading). 2001 Jan;147(Pt 1):63-73. doi: 10.1099/00221287-147-1-63. Microbiology (Reading). 2001. PMID: 11160801
-
Regulation of the expression of gas vesicle genes in Haloferax mediterranei: interaction of the two regulatory proteins GvpD and GvpE.Mol Microbiol. 2003 Aug;49(3):783-94. doi: 10.1046/j.1365-2958.2003.03593.x. Mol Microbiol. 2003. PMID: 12864859
-
Recent Advances in the Study of Gas Vesicle Proteins and Application of Gas Vesicles in Biomedical Research.Life (Basel). 2022 Sep 19;12(9):1455. doi: 10.3390/life12091455. Life (Basel). 2022. PMID: 36143491 Free PMC article. Review.
Cited by
-
Modification of PEG reduces the immunogenicity of biosynthetic gas vesicles.Front Bioeng Biotechnol. 2023 Mar 6;11:1128268. doi: 10.3389/fbioe.2023.1128268. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36949883 Free PMC article.
-
Regulation of sedimentation rate shapes the evolution of multicellularity in a close unicellular relative of animals.PLoS Biol. 2022 Mar 29;20(3):e3001551. doi: 10.1371/journal.pbio.3001551. eCollection 2022 Mar. PLoS Biol. 2022. PMID: 35349578 Free PMC article.
-
Biosynthetic Gas Vesicles Combined with Focused Ultrasound for Blood-Brain Barrier Opening.Int J Nanomedicine. 2022 Dec 28;17:6759-6772. doi: 10.2147/IJN.S374039. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36597431 Free PMC article.
-
Bioengineering of air-filled protein nanoparticles by genetic and chemical functionalization.J Nanobiotechnology. 2023 Mar 25;21(1):108. doi: 10.1186/s12951-023-01866-7. J Nanobiotechnology. 2023. PMID: 36966297 Free PMC article.
-
Biogenic Imaging Contrast Agents.Adv Sci (Weinh). 2023 Sep;10(25):e2207090. doi: 10.1002/advs.202207090. Epub 2023 Jul 3. Adv Sci (Weinh). 2023. PMID: 37401173 Free PMC article. Review.
References
-
- Nelson-Sathi S., Dagan T., Landan G., Janssen A., Steel M., McInerney J.O., Deppenmeier U., Martin W.F. Acquisition of 1000 eubacterial genes physiologically transformed a methanogen at the origin of haloarchaea. Proc. Natl. Acad. Sci. USA. 2012;109:20537–20542. doi: 10.1073/pnas.1209119109. - DOI - PMC - PubMed
-
- Klebahn H. Gasvakuolen, ein Bestandteil der Zellen der wasserblütenbildenden Phycochromaceen. Flora (Jena) 1895;80:241–282. (In German)
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

