Impaired bone homeostasis in amyotrophic lateral sclerosis mice with muscle atrophy
- PMID: 25648889
- PMCID: PMC4375466
- DOI: 10.1074/jbc.M114.603985
Impaired bone homeostasis in amyotrophic lateral sclerosis mice with muscle atrophy
Abstract
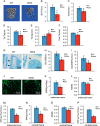
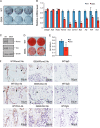
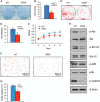
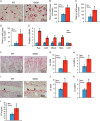
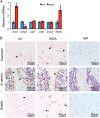
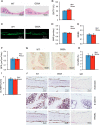
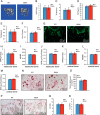
There is an intimate relationship between muscle and bone throughout life. However, how alterations in muscle functions in disease impact bone homeostasis is poorly understood. Amyotrophic lateral sclerosis (ALS) is a neuromuscular disease characterized by progressive muscle atrophy. In this study we analyzed the effects of ALS on bone using the well established G93A transgenic mouse model, which harbors an ALS-causing mutation in the gene encoding superoxide dismutase 1. We found that 4-month-old G93A mice with severe muscle atrophy had dramatically reduced trabecular and cortical bone mass compared with their sex-matched wild type (WT) control littermates. Mechanically, we found that multiple osteoblast properties, such as the formation of osteoprogenitors, activation of Akt and Erk1/2 pathways, and osteoblast differentiation capacity, were severely impaired in primary cultures and bones from G93A relative to WT mice; this could contribute to reduced bone formation in the mutant mice. Conversely, osteoclast formation and bone resorption were strikingly enhanced in primary bone marrow cultures and bones of G93A mice compared with WT mice. Furthermore, sclerostin and RANKL expression in osteocytes embedded in the bone matrix were greatly up-regulated, and β-catenin was down-regulated in osteoblasts from G93A mice when compared with those of WT mice. Interestingly, calvarial bone that does not load and long bones from 2-month-old G93A mice without muscle atrophy displayed no detectable changes in parameters for osteoblast and osteoclast functions. Thus, for the first time to our knowledge, we have demonstrated that ALS causes abnormal bone remodeling and defined the underlying molecular and cellular mechanisms.
Keywords: Amyotrophic Lateral Sclerosis (ALS) (Lou Gehrig Disease); Bone; Muscle Atrophy; Osteoblast; Osteoclast.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Carboxyl-terminal modulator protein regulates Akt signaling during skeletal muscle atrophy in vitro and a mouse model of amyotrophic lateral sclerosis.Sci Rep. 2019 Mar 8;9(1):3920. doi: 10.1038/s41598-019-40553-2. Sci Rep. 2019. PMID: 30850672 Free PMC article.
-
Human skeletal muscle atrophy in amyotrophic lateral sclerosis reveals a reduction in Akt and an increase in atrogin-1.FASEB J. 2006 Mar;20(3):583-5. doi: 10.1096/fj.05-5249fje. Epub 2006 Jan 17. FASEB J. 2006. PMID: 16507768
-
Progressive impairment of CaV1.1 function in the skeletal muscle of mice expressing a mutant type 1 Cu/Zn superoxide dismutase (G93A) linked to amyotrophic lateral sclerosis.Skelet Muscle. 2016 Jun 23;6:24. doi: 10.1186/s13395-016-0094-6. eCollection 2016. Skelet Muscle. 2016. PMID: 27340545 Free PMC article.
-
Potential Roles of the WNT Signaling Pathway in Amyotrophic Lateral Sclerosis.Cells. 2021 Apr 8;10(4):839. doi: 10.3390/cells10040839. Cells. 2021. PMID: 33917816 Free PMC article. Review.
-
Muscle Involvement in Amyotrophic Lateral Sclerosis: Understanding the Pathogenesis and Advancing Therapeutics.Biomolecules. 2023 Oct 26;13(11):1582. doi: 10.3390/biom13111582. Biomolecules. 2023. PMID: 38002264 Free PMC article. Review.
Cited by
-
Dysregulated mitochondrial Ca2+ and ROS signaling in skeletal muscle of ALS mouse model.Arch Biochem Biophys. 2019 Mar 15;663:249-258. doi: 10.1016/j.abb.2019.01.024. Epub 2019 Jan 22. Arch Biochem Biophys. 2019. PMID: 30682329 Free PMC article. Review.
-
Muscle-Bone Crosstalk in Amyotrophic Lateral Sclerosis.Curr Osteoporos Rep. 2015 Oct;13(5):274-9. doi: 10.1007/s11914-015-0281-0. Curr Osteoporos Rep. 2015. PMID: 26223903 Review.
-
Amyotrophic lateral sclerosis and osteoporosis: a two-sample Mendelian randomization study.Front Aging Neurosci. 2023 Dec 14;15:1305040. doi: 10.3389/fnagi.2023.1305040. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38161591 Free PMC article.
-
Antibody-Based PET Imaging of Misfolded Superoxide Dismutase 1 in an Amyotrophic Lateral Sclerosis Mouse Model.J Nucl Med. 2025 Jan 3;66(1):130-135. doi: 10.2967/jnumed.124.268343. J Nucl Med. 2025. PMID: 39667814 Free PMC article.
-
Wnt/β-catenin signaling pathway as an important mediator in muscle and bone crosstalk: A systematic review.J Orthop Translat. 2024 Jun 20;47:63-73. doi: 10.1016/j.jot.2024.06.003. eCollection 2024 Jul. J Orthop Translat. 2024. PMID: 39007034 Free PMC article. Review.
References
-
- Pasinelli P., Brown R. H. (2006) Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 7, 710–723 - PubMed
-
- Borchelt D. R., Lee M. K., Slunt H. S., Guarnieri M., Xu Z. S., Wong P. C., Brown R. H., Jr., Price D. L., Sisodia S. S., Cleveland D. W. (1994) Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl. Acad. Sci. U.S.A. 91, 8292–8296 - PMC - PubMed
-
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. (1993) Mutations in Cu2+/Zn2+ superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 - PubMed
-
- McGoldrick P., Joyce P. I., Fisher E. M., Greensmith L. (2013) Rodent models of amyotrophic lateral sclerosis. Biochim. Biophys. Acta 1832, 1421–1436 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

