Tetanus toxin fragment C fused to flagellin makes a potent mucosal vaccine
- PMID: 25649002
- PMCID: PMC4313110
- DOI: 10.7774/cevr.2015.4.1.59
Tetanus toxin fragment C fused to flagellin makes a potent mucosal vaccine
Abstract
Purpose: Recombinant subunit vaccines provide safe and targeted protection against microbial infections. However, the protective efficacy of recombinant subunit vaccines tends to be less potent than the whole cell vaccines, especially when they are administered through mucosal routes. We have reported that a bacterial flagellin has strong mucosal adjuvant activity to induce protective immune responses. In this study, we tested whether FlaB could be used as a fusion partner of subunit vaccine for tetanus.
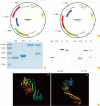
Materials and methods: We constructed fusion proteins consisted with tetanus toxin fragment C (TTFC), the nontoxic C-terminal portion of tetanus toxin, and a Toll-like receptor 5 agonist from Vibrio vulnificus (FlaB). Mice were intranasally administered with fusion protein and protective immune responses of the vaccinated mice were analyzed.
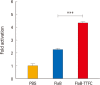
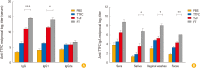
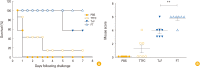
Results: FlaB-TTFC recombinant protein induced strong tetanus-specific antibody responses in both systemic and mucosal compartments and prolonged the survival of mice after challenge with a supra-lethal dose of tetanus toxin.
Conclusion: This study establishes FlaB as a successful fusion partner for recombinant subunit tetanus vaccine applicable through mucosal route, and it further endorses our previous observations that FlaB could be a stable adjuvant partner for mucosal vaccines.
Keywords: Adjuvant; Flagellin; Subunit vaccine; Tetanus toxin fragment C.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Plotkin SA, Plotkin SL. The development of vaccines: how the past led to the future. Nat Rev Microbiol. 2011;9:889–893. - PubMed
-
- Unnikrishnan M, Rappuoli R, Serruto D. Recombinant bacterial vaccines. Curr Opin Immunol. 2012;24:337–342. - PubMed
-
- Campbell JD, Clement KH, Wasserman SS, Donegan S, Chrisley L, Kotloff KL. Safety, reactogenicity and immunogenicity of a recombinant protective antigen anthrax vaccine given to healthy adults. Hum Vaccin. 2007;3:205–211. - PubMed
-
- Pizza M, Covacci A, Bartoloni A, et al. Mutants of pertussis toxin suitable for vaccine development. Science. 1989;246:497–500. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

