Higher Risk of Infections with PI3K-AKT-mTOR Pathway Inhibitors in Patients with Advanced Solid Tumors on Phase I Clinical Trials
- PMID: 25649020
- PMCID: PMC4401558
- DOI: 10.1158/1078-0432.CCR-14-2424
Higher Risk of Infections with PI3K-AKT-mTOR Pathway Inhibitors in Patients with Advanced Solid Tumors on Phase I Clinical Trials
Abstract
Purpose: Novel antitumor therapies against the PI3K-AKT-mTOR pathway are increasingly used to treat cancer, either as single agents or in combination with chemotherapy or other targeted therapies. Although these agents are not known to be myelosuppressive, an increased risk of infection has been reported with rapamycin analogues. However, the risk of infection with new inhibitors of this pathway such as PI3K, AKT, mTORC 1/2, or multikinase inhibitors is unknown.
Experimental design: In this retrospective case-control study, we determined the incidence of infection in a group of 432 patients who were treated on 15 phase I clinical trials involving PI3K-AKT-mTOR pathway inhibitors (cases) versus a group of 100 patients on 10 phase I clinical trials of single agent non-PI3K-AKT-mTOR pathway inhibitors (controls) which did not involve conventional cytotoxic agents. We also collected data from 42 patients who were treated with phase I trials of combinations of PI3K-AKT-mTOR inhibitors and MEK inhibitors and 24 patients with combinations of PI3K-AKT-mTOR inhibitors and cytotoxic chemotherapies.
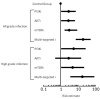
Results: The incidence of all grade infection was significantly higher with all single-agent PI3K-AKT-mTOR inhibitors compared with the control group [27% vs. 8%, respectively, OR, 4.26; 95% confidence intervals (CI), 1.9-9.1, P = 0.0001]. The incidence of grade 3 and 4 infection was also significantly higher with PI3K-AKT-mTOR inhibitors compared with the control group (10.3% vs. 3%, OR, 3.74; 95% CI, 1.1-12.4; P = 0.02). Also, the combination of PI3K-AKT-mTOR inhibitors and chemotherapy was associated with a significantly higher incidence of all grade (OR, 4.79; 95% CI, 2.0-11.2; P = 0.0001) and high-grade (OR, 2.87; 95% CI, 1.0-7.6; P = 0.03) infection when compared with single-agent PI3K-AKT-mTOR inhibitors.
Conclusions: Inhibitors of the PI3K-AKT-mTOR pathway can be associated with a higher risk of infection. Combinations of PI3K-AKT-mTOR inhibitors and cytotoxic chemotherapy significantly increase the risk of infection. This should be taken into consideration during the design and conduct of trials involving PI3K-AKT-mTOR pathway inhibitors, particularly when combined with chemotherapy or myelosuppressive agents.
©2015 American Association for Cancer Research.
Figures
Similar articles
-
Hyperglycemia and Phosphatidylinositol 3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin (PI3K/AKT/mTOR) Inhibitors in Phase I Trials: Incidence, Predictive Factors, and Management.Oncologist. 2016 Jul;21(7):855-60. doi: 10.1634/theoncologist.2015-0248. Epub 2016 May 5. Oncologist. 2016. PMID: 27151652 Free PMC article. Clinical Trial.
-
Complications of hyperglycaemia with PI3K-AKT-mTOR inhibitors in patients with advanced solid tumours on Phase I clinical trials.Br J Cancer. 2015 Dec 1;113(11):1541-7. doi: 10.1038/bjc.2015.373. Epub 2015 Nov 10. Br J Cancer. 2015. PMID: 26554652 Free PMC article.
-
Impact of patient ethnicity on the metabolic and immunologic effects of PI3K-mTOR pathway inhibition in patients with solid tumor malignancies.Cancer Chemother Pharmacol. 2014 Aug;74(2):359-65. doi: 10.1007/s00280-014-2510-0. Epub 2014 Jun 17. Cancer Chemother Pharmacol. 2014. PMID: 24934865 Free PMC article.
-
The effectiveness of monotherapy with PI3K/AKT/mTOR pathway inhibitors in ovarian cancer: A meta-analysis.Gynecol Oncol. 2021 Nov;163(2):433-444. doi: 10.1016/j.ygyno.2021.07.008. Epub 2021 Jul 10. Gynecol Oncol. 2021. PMID: 34253390
-
PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside.Semin Cancer Biol. 2019 Dec;59:125-132. doi: 10.1016/j.semcancer.2019.07.009. Epub 2019 Jul 16. Semin Cancer Biol. 2019. PMID: 31323288 Review.
Cited by
-
Risk of Infectious Complications in Hemato-Oncological Patients Treated with Kinase Inhibitors.Biomark Insights. 2016 Apr 21;10(Suppl 3):55-68. doi: 10.4137/BMI.S22430. eCollection 2015. Biomark Insights. 2016. PMID: 27127405 Free PMC article. Review.
-
Immunomodulation and Disease Tolerance to Staphylococcus aureus.Pathogens. 2015 Nov 13;4(4):793-815. doi: 10.3390/pathogens4040793. Pathogens. 2015. PMID: 26580658 Free PMC article. Review.
-
Global Proteomics Revealed Klebsiella pneumoniae Induced Autophagy and Oxidative Stress in Caenorhabditis elegans by Inhibiting PI3K/AKT/mTOR Pathway during Infection.Front Cell Infect Microbiol. 2017 Sep 6;7:393. doi: 10.3389/fcimb.2017.00393. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28932706 Free PMC article.
-
Molecular Pathways: Increased Susceptibility to Infection Is a Complication of mTOR Inhibitor Use in Cancer Therapy.Clin Cancer Res. 2016 Jan 15;22(2):277-83. doi: 10.1158/1078-0432.CCR-14-3239. Epub 2015 Nov 25. Clin Cancer Res. 2016. PMID: 26607598 Free PMC article. Review.
-
Hyperglycemia and Phosphatidylinositol 3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin (PI3K/AKT/mTOR) Inhibitors in Phase I Trials: Incidence, Predictive Factors, and Management.Oncologist. 2016 Jul;21(7):855-60. doi: 10.1634/theoncologist.2015-0248. Epub 2016 May 5. Oncologist. 2016. PMID: 27151652 Free PMC article. Clinical Trial.
References
-
- Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. - PubMed
-
- Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. - PubMed
-
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous