Safety and Immunogenicity of a Subvirion Monovalent Unadjuvanted Inactivated Influenza A(H3N2) Variant Vaccine in Healthy Persons ≥18 Years Old
- PMID: 25649171
- PMCID: PMC4539893
- DOI: 10.1093/infdis/jiv056
Safety and Immunogenicity of a Subvirion Monovalent Unadjuvanted Inactivated Influenza A(H3N2) Variant Vaccine in Healthy Persons ≥18 Years Old
Abstract
Background: Variant influenza A(H3N2) viruses (H3N2v) have transmitted recently from pigs to humans in the United States. Vaccines strategies are needed.
Methods: Healthy adults received 2 doses of subvirion H3N2v vaccine (15 µg of hemagglutinin/dose) 21 days apart in this open-label trial. Serum hemagglutination inhibition (HAI) and neutralizing (Neut) antibody (Ab) titers were measured before and 8 and 21 days after each dose. Memory B-cell (MBC) responses were assessed.
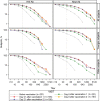
Results: Vaccine was well tolerated. A total of 40% of subjects had an HAI Ab titer of ≥40 before vaccination. Eight-seven percent (95% confidence interval [CI], 79%-93%) and 73% (95% CI, 63%-81%) of subjects 18-64 years old (98 subjects) and ≥65 years old (90 subjects), respectively, had an HAI titer of ≥40 21 days after dose 1 (P = .01); 51% (95% CI, 41%-61%) and 52% (95% CI, 41%-62%) of younger and older subjects, respectively, developed ≥4-fold rises in titer (P = not significant). Neut Ab response patterns were similar. Geometric mean titers were higher in younger subjects. Dose 2 provided no significant enhancement in responses. Cross-reactive MBCs were detected before vaccination and expanded after vaccination. Preexisting H3N2v-specific MBCs positively correlated with early increases in vaccine-induced Ab.
Conclusions: In most healthy adults, one 15-µg dose of vaccine elicited levels of HAI Abs associated with protection. Studies in children and elderly individuals are indicated to define the immunization needs of these groups.
Clinical trials registration: NCT01746082.
Keywords: H3N2 variant; immune responses; immunization; influenza; pandemic.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- CDC. Update: influenza A (H3N2)v transmission and guidelines—five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–4. - PubMed
-
- CDC. Swine-origin influenza A (H3N2) virus infection in two children—Indiana and Pennsylvania, July–August 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1213–5. - PubMed
-
- CDC. Limited human-to-human transmission of novel influenza A(H3N2) virus—Iowa, November 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1615–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN272200800006C/AI/NIAID NIH HHS/United States
- UL1TR000454/TR/NCATS NIH HHS/United States
- HHSN272200800006C/PHS HHS/United States
- HHSN272200800008C/AI/NIAID NIH HHS/United States
- HHSN272200800007C/PHS HHS/United States
- HHSN272200800002C/AI/NIAID NIH HHS/United States
- HHSN272200800002C/PHS HHS/United States
- T32 AI074492/AI/NIAID NIH HHS/United States
- HHSN272200800013C/AI/NIAID NIH HHS/United States
- HHSN272200800005C/AI/NIAID NIH HHS/United States
- HHSN272200800004C/PHS HHS/United States
- U54 TR001356/TR/NCATS NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- HHSN272200800004C/AI/NIAID NIH HHS/United States
- HHSN272200800008C/PHS HHS/United States
- HHSN272200800005C/PHS HHS/United States
- HHSN272200800013C/PHS HHS/United States
- HHSN272200800007C/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

