Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial
- PMID: 25649209
- PMCID: PMC4322191
- DOI: 10.1136/bmjopen-2014-006131
Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial
Abstract
Objective: To investigate the safety and efficacy of QMF149, a once-daily, fixed-dose combination of the long-acting β2-agonist (LABA) indacaterol maleate and inhaled corticosteroid (ICS) mometasone furoate (MF) for the treatment of persistent asthma. The hypothesis was that QMF149 would not increase the risk of serious asthma exacerbations.
Setting: 174 research centres in nine countries.
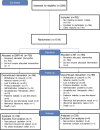
Participants: 1519 adolescents and adults with persistent asthma who were treated or qualified for treatment with combination LABA/ICS were randomised, and 1508 were included in the intention-to-treat analysis.
Intervention: Patients were randomised to QMF149 (indacaterol maleate 500 µg/MF 400 µg) or MF (400 µg) once daily via Twisthaler inhalation device in a double-blind, parallel-group study for 6-21 months.
Primary and secondary outcome measures: The primary end point was time to first serious asthma exacerbation (resulting in hospitalisation, intubation or death). The key secondary end point was annual rate of exacerbations requiring systemic corticosteroids.
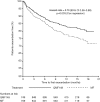
Results: Treatment with QMF149 resulted in no significant difference in time to first serious exacerbation compared to MF (2 (0.3%) vs 6 events (0.8%); difference -0.52 percentage point; 95% CI -1.25 to 0.21, p=0.160, HR=0.31; 95% CI 0.06 to 1.54, p=0.151). QMF149 significantly reduced the annual rate of exacerbations requiring systemic corticosteroids (rate ratio=0.71; 95% CI 0.55 to 0.90, p=0.005). Proportions of patients experiencing adverse events were similar across groups (74.0% in the QMF149 group and 73.4% in the MF group). Serious adverse events occurred in 4% and 5.8% of patients in the QMF149 and MF groups, respectively.
Conclusions: No significant difference was observed in the primary outcome of time to first serious asthma exacerbation in patients treated with QMF149 compared with patients treated with MF. Long-term treatment with QMF149 once daily had a favourable safety/efficacy profile in adolescent and adult patients with persistent asthma.
Trial registration number: ClinicalTrials.gov; NCT00941798.
Keywords: CLINICAL PHARMACOLOGY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2012. http://www.ginasthma.org/local/uploads/files/GINA_Report_March13.pdf (accessed Jun 2014).
-
- NHLBI. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Full report National Institutes of Health, National Heart Lung and Blood Institute, 2007. http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines... (accessed 28 Jan 2015).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical