Inhibition of IAPP aggregation by insulin depends on the insulin oligomeric state regulated by zinc ion concentration
- PMID: 25649462
- PMCID: PMC4316164
- DOI: 10.1038/srep08240
Inhibition of IAPP aggregation by insulin depends on the insulin oligomeric state regulated by zinc ion concentration
Abstract
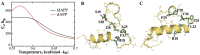
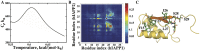
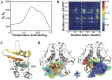
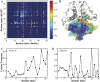
While islet amyloid polypeptide (IAPP) aggregation is associated with β-cell death in type-II diabetes (T2D), environmental elements of β-cell granules - e.g. high concentrations of insulin and Zn(2+) - inhibit IAPP aggregation in healthy individuals. The inhibition by insulin is experimentally known, but the role of Zn(2+) is controversial as both correlations and anti-correlations at the population level are observed between T2D risk and the activity of a β-cell specific zinc ion transporter, ZnT8. Since Zn(2+) concentration determines insulin oligomer equilibrium, we computationally investigated interactions of IAPP with different insulin oligomers and compared with IAPP homodimer formation. We found that IAPP binding with insulin oligomers competes with the formation of both higher-molecular-weight insulin oligomers and IAPP homodimers. Therefore, zinc deficiency due to loss-of-function ZnT8 mutations shifts insulin oligomer equilibrium toward zinc-free monomers and dimers, which bind IAPP monomers more efficiently compared to zinc-bound hexamers. The hetero-molecular complex formation prevents IAPP from self-association and subsequent aggregation, reducing T2D risk.
Figures
Similar articles
-
Promotion or Inhibition of Islet Amyloid Polypeptide Aggregation by Zinc Coordination Depends on Its Relative Concentration.Biochemistry. 2015 Dec 22;54(50):7335-44. doi: 10.1021/acs.biochem.5b00891. Epub 2015 Dec 4. Biochemistry. 2015. PMID: 26603575
-
Investigation of the effects of two major secretory granules components, insulin and zinc, on human-IAPP amyloid aggregation and membrane damage.Chem Phys Lipids. 2021 Jul;237:105083. doi: 10.1016/j.chemphyslip.2021.105083. Epub 2021 Apr 19. Chem Phys Lipids. 2021. PMID: 33887213
-
Causative factors for formation of toxic islet amyloid polypeptide oligomer in type 2 diabetes mellitus.Clin Interv Aging. 2015 Nov 19;10:1873-9. doi: 10.2147/CIA.S95297. eCollection 2015. Clin Interv Aging. 2015. PMID: 26604727 Free PMC article. Review.
-
Insulin, islet amyloid polypeptide and C-peptide interactions evaluated by mass spectrometric analysis.Rapid Commun Mass Spectrom. 2014 Jan 30;28(2):178-84. doi: 10.1002/rcm.6772. Rapid Commun Mass Spectrom. 2014. PMID: 24338965
-
A mechanistic approach for islet amyloid polypeptide aggregation to develop anti-amyloidogenic agents for type-2 diabetes.Biochimie. 2011 May;93(5):793-805. doi: 10.1016/j.biochi.2010.12.012. Epub 2011 Jan 5. Biochimie. 2011. PMID: 21215287 Review.
Cited by
-
Stabilizing Off-pathway Oligomers by Polyphenol Nanoassemblies for IAPP Aggregation Inhibition.Sci Rep. 2016 Jan 14;6:19463. doi: 10.1038/srep19463. Sci Rep. 2016. PMID: 26763863 Free PMC article.
-
Modulating protein amyloid aggregation with nanomaterials.Environ Sci Nano. 2017 Sep 1;4(9):1772-1783. doi: 10.1039/C7EN00436B. Epub 2017 Jul 28. Environ Sci Nano. 2017. PMID: 29230295 Free PMC article.
-
Zinc and pH modulate the ability of insulin to inhibit aggregation of islet amyloid polypeptide.Commun Biol. 2024 Jun 27;7(1):776. doi: 10.1038/s42003-024-06388-y. Commun Biol. 2024. PMID: 38937578 Free PMC article.
-
Liquid-liquid phase separation of type II diabetes-associated IAPP initiates hydrogelation and aggregation.Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12050-12061. doi: 10.1073/pnas.1916716117. Epub 2020 May 15. Proc Natl Acad Sci U S A. 2020. PMID: 32414928 Free PMC article.
-
Zinc-coordination and C-peptide complexation: a potential mechanism for the endogenous inhibition of IAPP aggregation.Chem Commun (Camb). 2017 Aug 22;53(68):9394-9397. doi: 10.1039/c7cc04291d. Chem Commun (Camb). 2017. PMID: 28745731 Free PMC article.
References
-
- Westermark P., Andersson A. & Westermark G. T. Is aggregated IAPP a cause of beta-cell failure in transplanted human pancreatic islets? Curr. Diab. Rep. 5, 184–188 (2005). - PubMed
-
- Konarkowska B., Aitken J. F., Kistler J., Zhang S. & Cooper G. J. S. The aggregation potential of human amylin determines its cytotoxicity towards islet β-cells. FEBS J. 273, 3614–3624 (2006). - PubMed
-
- Hebda J. A. & Miranker A. D. The Interplay of Catalysis and Toxicity by Amyloid Intermediates on Lipid Bilayers: Insights from Type II Diabetes. Annu. Rev. Biophys. 38, 125–152 (2009). - PubMed
-
- Sladek R. et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445, 881–885 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

