BH3 Inhibitor Sensitivity and Bcl-2 Dependence in Primary Acute Lymphoblastic Leukemia Cells
- PMID: 25649768
- PMCID: PMC4383710
- DOI: 10.1158/0008-5472.CAN-14-1849
BH3 Inhibitor Sensitivity and Bcl-2 Dependence in Primary Acute Lymphoblastic Leukemia Cells
Abstract
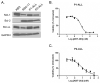
BH3 mimetic drugs may be useful to treat acute lymphoblastic leukemia (ALL) but the sensitivity of primary tumor cells has not been fully evaluated. Here, B-lineage ALL cell cultures derived from a set of primary tumors were studied with respect to sensitivity to the BH3 mimetics ABT-263 and ABT-199 and to Bcl-2 dependence and function. These ALL cells each expressed high levels of Bcl-2 and exhibited great sensitivity to ABT-263 and ABT-199, which induced rapid apoptotic cell death. BH3 profiling indicated that the ALL cultures were Bcl-2 dependent. Coimmunoprecipitation studies revealed a multifaceted role for Bcl-2 in binding proapoptotic partners including Bax, Bak, Bik, and Bim. ABT-263 disrupted Bcl-2:Bim interaction in cells. Mcl-1 overexpression rendered ALL cells resistant to ABT-263 and ABT-199, with Mcl-1 assuming the role of Bcl-2 in binding Bim. Freshly isolated pediatric ALL blasts also expressed high levels of Bcl-2 and exhibited high sensitivity to Bcl-2 inhibition by the BH3 mimetic compounds. Overall, our results showed that primary ALL cultures were both more sensitive to BH3 mimetics and more uniform in their response than established ALL cell lines that have been evaluated previously. Furthermore, the primary cell model characterized here offers a powerful system for preclinical testing of novel drugs and drug combinations to treat ALL.
©2015 American Association for Cancer Research.
Figures



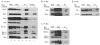

References
-
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews Molecular cell biology. 2008;9(1):47–59. - PubMed
-
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature reviews Molecular cell biology. 2014;15(1):49–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

