The expression dynamics of transforming growth factor-β/Smad signaling in the liver fibrosis experimentally caused by Clonorchis sinensis
- PMID: 25649869
- PMCID: PMC4329204
- DOI: 10.1186/s13071-015-0675-y
The expression dynamics of transforming growth factor-β/Smad signaling in the liver fibrosis experimentally caused by Clonorchis sinensis
Abstract
Background: Liver fibrosis is a hallmark of clonorchiasis suffered by millions people in Eastern Asian countries. Recent studies showed that the activation of TGF-β/Smad signaling pathway can potently regulate the hepatic fibrogenesis including Schistosoma spp. and Echinococcus multilocularis-caused liver fibrosis. However, little is known to date about the expression of transforming growth factor-β (TGF-β) and other molecules in TGF-β/Smad signaling pathway which may play an important role in hepatic fibrosis caused by C. sinensis.
Methods: A total of 24 mice were individually infected orally with 45 metacercariae, both experimental mice and mocked-infected control mice were anesthetized at 4 week post-infection (wk p.i.), 8 wk p.i. and 16 wk p.i., respectively. For each time-point, the liver and serum from each animal were collected to analyze histological findings and various fibrotic parameters including TGF-β₁, TGF-β receptors and down-stream Smads activation, as well as fibrosis markers expression.
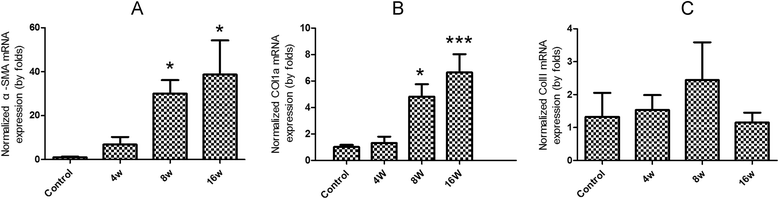
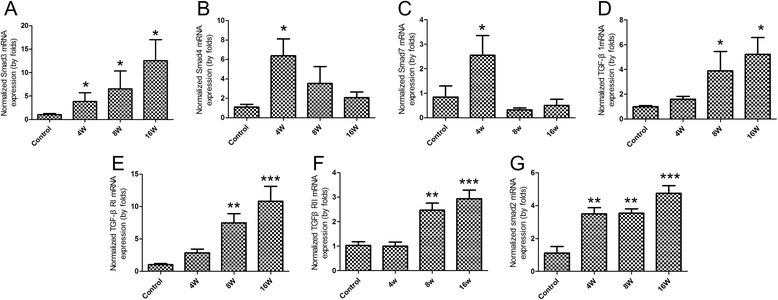
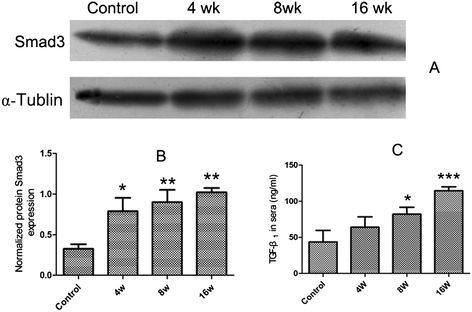
Results: The results showed that collagen deposition indicated by hydroxyproline content and Masson's trichrome staining was increased gradually with the development of infection. The expression of collagen type α1 (Col1a) mRNA transcripts was steadily increased during the whole infection. The mRNA levels of Smad2, Smad3 as well as the protein of Smad3 in the liver of C. sinensis-infected mice were increased after 4 wk p.i. (P < 0.05, compared with normal control) whereas the TGF-β₁, TGF-β type I receptor (TGFβRI) and TGF-β type II receptor (TGFβRII) mRNA expression in C. sinensis-infected mice were higher than those of normal control mice after 8 wk p.i. (P < 0.05). However, the gene expression of Smad4 and Smad7 were peaked at 4 wk p.i. (P < 0.05), and thereafter dropped to the basal level at 8 wk p.i., and 16 wk p.i., respectively. The concentrations of TGF-β₁ in serum in the C. sinensis-infected mice at 8 wk p.i. and 16 wk p.i (P < 0.05) were significantly higher than those in the control mice.
Conclusions: The results of the present study indicated for the first time that the activation of TGF-β/Smad signaling pathway might contribute to the synthesis of collagen type I which leads to liver fibrosis caused by C. sinensis.
Figures

References
-
- Fang YY, Chen YD, Li XM, Wu J, Zhang QM, Ruan CW. Current prevalence of Clonorchis sinensis infection in endemic areas of China. ZhongguoJi Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2008, 26:99–103, 109. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

