Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons
- PMID: 25649872
- PMCID: PMC4406823
- DOI: 10.1111/jnc.13040
Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons
Abstract
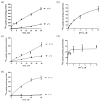
The molecular mechanisms of iron trafficking in neurons have not been elucidated. In this study, we characterized the expression and localization of ferrous iron transporters Zip8, Zip14 and divalent metal transporter 1 (DMT1), and ferrireductases Steap2 and stromal cell-derived receptor 2 in primary rat hippocampal neurons. Steap2 and Zip8 partially co-localize, indicating these two proteins may function in Fe(3+) reduction prior to Fe(2+) permeation. Zip8, DMT1, and Steap2 co-localize with the transferrin receptor/transferrin complex, suggesting they may be involved in transferrin receptor/transferrin-mediated iron assimilation. In brain interstitial fluid, transferring-bound iron (TBI) and non-transferrin-bound iron (NTBI) exist as potential iron sources. Primary hippocampal neurons exhibit significant iron uptake from TBI (Transferrin-(59) Fe(3+)) and NTBI, whether presented as (59) Fe(2+) -citrate or (59) Fe(3+) -citrate; reductase-independent (59) Fe(2+) uptake was the most efficient uptake pathway of the three. Kinetic analysis of Zn(2+) inhibition of Fe(2+) uptake indicated that DMT1 plays only a minor role in the uptake of NTBI. In contrast, localization and knockdown data indicate that Zip8 makes a major contribution. Data suggest also that cell accumulation of (59) Fe from TBI relies at least in part on an endocytosis-independent pathway. These data suggest that Zip8 and Steap2 play a major role in iron accumulation from NTBI and TBI by hippocampal neurons. Analysis of the expression and localization of known iron uptake transporters demonstrated that Zip8 makes a major contribution to iron accumulation in primary cultures of rat embryonic hippocampal neurons. These cells exhibit uptake pathways for ferrous and ferric iron (non-transferrin-bound iron, NTBI in figure) and for transferrin-bound iron; the ferrireductases Steap2 and SDR2 support the uptake of ferric iron substrates. Zip8 and Steap2 are strongly expressed in the plasma membrane of both soma and processes, implying a crucial role in iron accumulation from NTBI and transferrin-bound iron (TBI) by hippocampal neurons.
Keywords: NTBI; TBI; Zip8; ferrireductase; permease; primary hippocampal neurons.
© 2015 International Society for Neurochemistry.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
Iron uptake by ZIP8 and ZIP14 in human proximal tubular epithelial cells.Biometals. 2019 Apr;32(2):211-226. doi: 10.1007/s10534-019-00183-7. Epub 2019 Feb 26. Biometals. 2019. PMID: 30806852 Free PMC article.
-
Hepatocyte divalent metal-ion transporter-1 is dispensable for hepatic iron accumulation and non-transferrin-bound iron uptake in mice.Hepatology. 2013 Aug;58(2):788-98. doi: 10.1002/hep.26401. Epub 2013 Jul 1. Hepatology. 2013. PMID: 23508576 Free PMC article.
-
Non-transferrin-bound iron uptake in Belgrade and normal rat erythroid cells.J Cell Physiol. 1999 Mar;178(3):349-58. doi: 10.1002/(SICI)1097-4652(199903)178:3<349::AID-JCP9>3.0.CO;2-R. J Cell Physiol. 1999. PMID: 9989781
-
A holistic view of mammalian (vertebrate) cellular iron uptake.Metallomics. 2020 Sep 23;12(9):1323-1334. doi: 10.1039/d0mt00065e. Metallomics. 2020. PMID: 32766655 Free PMC article. Review.
-
Non-transferrin-bound iron transporters.Free Radic Biol Med. 2019 Mar;133:101-111. doi: 10.1016/j.freeradbiomed.2018.10.413. Epub 2018 Oct 12. Free Radic Biol Med. 2019. PMID: 30316781 Review.
Cited by
-
Mechanisms and regulation of iron trafficking across the capillary endothelial cells of the blood-brain barrier.Front Mol Neurosci. 2015 Jul 14;8:31. doi: 10.3389/fnmol.2015.00031. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26236187 Free PMC article. Review.
-
Molecular and pathophysiological aspects of metal ion uptake by the zinc transporter ZIP8 (SLC39A8).Toxicol Res (Camb). 2016 Feb 18;5(4):987-1002. doi: 10.1039/c5tx00424a. eCollection 2016 Jul 1. Toxicol Res (Camb). 2016. PMID: 30090406 Free PMC article. Review.
-
A conjoint analysis of bulk RNA-seq and single-nucleus RNA-seq for revealing the role of ferroptosis and iron metabolism in ALS.Front Neurosci. 2023 Mar 2;17:1113216. doi: 10.3389/fnins.2023.1113216. eCollection 2023. Front Neurosci. 2023. PMID: 36937665 Free PMC article.
-
The Dual Role of Hepcidin in Brain Iron Load and Inflammation.Front Neurosci. 2018 Oct 15;12:740. doi: 10.3389/fnins.2018.00740. eCollection 2018. Front Neurosci. 2018. PMID: 30374287 Free PMC article. Review.
-
Inhibition of STEAP1 ameliorates inflammation and ferroptosis of acute lung injury caused by sepsis in LPS-induced human pulmonary microvascular endothelial cells.Mol Biol Rep. 2023 Jul;50(7):5667-5674. doi: 10.1007/s11033-023-08403-7. Epub 2023 May 20. Mol Biol Rep. 2023. PMID: 37209327
References
-
- Altamura S, Muckenthaler MU. Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis. Journal of Alzheimer’s disease : JAD. 2009;16:879–895. - PubMed
-
- Berczi A, Su D, Lakshminarasimhan M, Vargas A, Asard H. Heterologous expression and site-directed mutagenesis of an ascorbate-reducible cytochrome b561. Archives of biochemistry and biophysics. 2005;443:82–92. - PubMed
-
- Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. American journal of physiology. Lung cellular and molecular physiology. 2008;294:L1127–1136. - PubMed
-
- Bleijenberg BG, Van Eijk HG, Leijnse B. The determination of non-heme iron and transferrin in cerebrospinal fluid. Clinica chimica acta; international journal of clinical chemistry. 1971;31:277–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

