Development, characterization, and in vitro biological performance of fluconazole-loaded microemulsions for the topical treatment of cutaneous leishmaniasis
- PMID: 25650054
- PMCID: PMC4306376
- DOI: 10.1155/2015/396894
Development, characterization, and in vitro biological performance of fluconazole-loaded microemulsions for the topical treatment of cutaneous leishmaniasis
Abstract
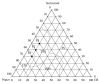
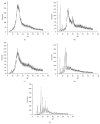
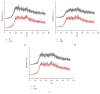
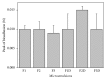
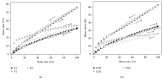
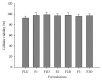
Cutaneous leishmaniasis (CL) is a resistant form of leishmaniasis that is caused by a parasite belonging to the genus Leishmania. FLU-loaded microemulsions (MEs) were developed by phase diagram for topical administration of fluconazole (FLU) as prominent alternative to combat CL. Three MEs called F1, F2, and F3 (F1-60% 50 M phosphate buffer at pH 7.4 (PB) as aqueous phase, 10% cholesterol (CHO) as oil phase, and 30% soy phosphatidylcholine/oil polyoxyl-60 hydrogenated castor oil/sodium oleate (3/8/6) (S) as surfactant; F2-50% PB, 10% CHO, and 40% S; F3-40% PB, 10% CHO, and 50 % S) were characterized by droplet size analysis, zeta potential analysis, X-ray diffraction, continuous flow, texture profile analysis, and in vitro bioadhesion. MEs presented pseudoplastic flow and thixotropy was dependent on surfactant concentration. Droplet size was not affected by FLU. FLU-loaded MEs improved the FLU safety profile that was evaluated using red cell haemolysis and in vitro cytotoxicity assays with J-774 mouse macrophages. FLU-unloaded MEs did not exhibit leishmanicidal activity that was performed using MTT colourimetric assays; however, FLU-loaded MEs exhibited activity. Therefore, these MEs have potential to modulate FLU action, being a promising platform for drug delivery systems to treat CL.
Figures
References
-
- Reis S. R., Gomes L. H. M., Ferreira N. M., et al. Ocorrência de flebotomíneos (Diptera: Psychodidae: Phlebotominae) no ambiente peridomiciliar em área de foco de transmissão de leishmaniose tegumentar no município de Manaus, Amazonas. Acta Amazonica. 2013;43(1):121–123. doi: 10.1590/s0044-59672013000100016. - DOI
-
- Shaw J. J., Lainson R. Leishmaniasis in Brazil: X. Some observations on intradermal reactions to different trypanosomatid antigens of patients suffering from cutaneous and mucocutaneous leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1975;69(3):323–335. doi: 10.1016/0035-9203(75)90127-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

