Differential effects of leptin and adiponectin in endothelial angiogenesis
- PMID: 25650072
- PMCID: PMC4310451
- DOI: 10.1155/2015/648239
Differential effects of leptin and adiponectin in endothelial angiogenesis
Abstract
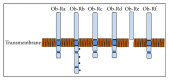
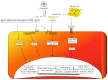
Obesity is a major health burden with an increased risk of cardiovascular morbidity and mortality. Endothelial dysfunction is pivotal to the development of cardiovascular disease (CVD). In relation to this, adipose tissue secreted factors termed "adipokines" have been reported to modulate endothelial dysfunction. In this review, we focus on two of the most abundant circulating adipokines, that is, leptin and adiponectin, in the development of endothelial dysfunction. Leptin has been documented to influence a multitude of organ systems, that is, central nervous system (appetite regulation, satiety factor) and cardiovascular system (endothelial dysfunction leading to atherosclerosis). Adiponectin, circulating at a much higher concentration, exists in different molecular weight forms, essentially made up of the collagenous fraction and a globular domain, the latter being investigated minimally for its involvement in proinflammatory processes including activation of NF-κβ and endothelial adhesion molecules. The opposing actions of the two forms of adiponectin in endothelial cells have been recently demonstrated. Additionally, a local and systemic change to multimeric forms of adiponectin has gained importance. Thus detailed investigations on the potential interplay between these adipokines would likely result in better understanding of the missing links connecting CVD, adipokines, and obesity.
Figures

References
-
- Gordon E. S. Non-esterified fatty acids in blood of obese and lean subjects. The American Journal of Clinical Nutrition. 1960;8:740–747.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

