From repair to regeneration: biomaterials to reprogram the meniscus wound microenvironment
- PMID: 25650096
- PMCID: PMC4380775
- DOI: 10.1007/s10439-015-1249-z
From repair to regeneration: biomaterials to reprogram the meniscus wound microenvironment
Abstract
When the field of tissue engineering first arose, scaffolds were conceived of as inert three-dimensional structures whose primary function was to support cellularity and tissue growth. Since then, advances in scaffold and biomaterial design have evolved to not only guide tissue formation, but also to interact dynamically with and manipulate the wound environment. At present, these efforts are being directed towards strategies that directly address limitations in endogenous wound repair, with the goal of reprogramming the local wound environment (and the cells within that locality) from a state that culminates in an inferior tissue repair into a state in which functional regeneration is achieved. This review will address this approach with a focus on recent advances in scaffold design towards the resolution of tears of the knee meniscus as a case example. The inherent limitations to endogenous repair will be discussed, as will specific examples of how biomaterials are being designed to overcome these limitations. Examples will include design of fibrous scaffolds that promote colonization by modulating local extracellular matrix density and delivering recruitment factors. Furthermore, we will discuss scaffolds that are themselves modulated by the wound environment to alter porosity and modulate therapeutic release through precise coordination of scaffold degradation. Finally, we will close with emerging concepts in local control of cell mechanics to improve interstitial cell migration and so advance repair. Overall, these examples will illustrate how emergent features within a biomaterial can be tuned to manipulate and harness the local tissue microenvironment in order to promote robust regeneration.
Figures





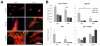
 denotes significance (p<0.05) compared to other RGD densities within the same fiber stiffness condition. Adapted with permission from [11].
denotes significance (p<0.05) compared to other RGD densities within the same fiber stiffness condition. Adapted with permission from [11].
Similar articles
-
[Recent progress of researches on scaffolds for tissue engineered meniscus].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010 Apr;27(2):458-62. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010. PMID: 20481339 Review. Chinese.
-
Advances in electrospun scaffolds for meniscus tissue engineering and regeneration.J Biomed Mater Res B Appl Biomater. 2022 Apr;110(4):923-949. doi: 10.1002/jbm.b.34952. Epub 2021 Oct 7. J Biomed Mater Res B Appl Biomater. 2022. PMID: 34619021 Review.
-
Animal models for meniscus repair and regeneration.J Tissue Eng Regen Med. 2015 May;9(5):512-27. doi: 10.1002/term.1760. Epub 2013 May 27. J Tissue Eng Regen Med. 2015. PMID: 23712959 Review.
-
Expanded human meniscus-derived cells in 3-D polymer-hyaluronan scaffolds for meniscus repair.Acta Biomater. 2012 Feb;8(2):677-85. doi: 10.1016/j.actbio.2011.10.007. Epub 2011 Oct 11. Acta Biomater. 2012. PMID: 22023746
-
Meniscus tissue engineering on the nanoscale: from basic principles to clinical application.J Knee Surg. 2009 Jan;22(1):45-59. doi: 10.1055/s-0030-1247727. J Knee Surg. 2009. PMID: 19216353 Free PMC article. Review.
Cited by
-
Meniscus-Derived Matrix Bioscaffolds: Effects of Concentration and Cross-Linking on Meniscus Cellular Responses and Tissue Repair.Int J Mol Sci. 2019 Dec 19;21(1):44. doi: 10.3390/ijms21010044. Int J Mol Sci. 2019. PMID: 31861690 Free PMC article.
-
The regenerative effect of different growth factors and platelet lysate on meniscus cells and mesenchymal stromal cells and proof of concept with a functionalized meniscus implant.J Tissue Eng Regen Med. 2021 Jul;15(7):648-659. doi: 10.1002/term.3218. Epub 2021 May 21. J Tissue Eng Regen Med. 2021. PMID: 33982442 Free PMC article.
-
Platelet-derived growth factor-coated decellularized meniscus scaffold for integrative healing of meniscus tears.Acta Biomater. 2018 Aug;76:126-134. doi: 10.1016/j.actbio.2018.06.021. Epub 2018 Jun 14. Acta Biomater. 2018. PMID: 29908335 Free PMC article.
-
Advances in Quantification of Meniscus Tensile Mechanics Including Nonlinearity, Yield, and Failure.J Biomech Eng. 2016 Feb;138(2):021002. doi: 10.1115/1.4032354. J Biomech Eng. 2016. PMID: 26720401 Free PMC article.
-
Large Animal Models of Meniscus Repair and Regeneration: A Systematic Review of the State of the Field.Tissue Eng Part C Methods. 2017 Nov;23(11):661-672. doi: 10.1089/ten.TEC.2017.0080. Epub 2017 Aug 4. Tissue Eng Part C Methods. 2017. PMID: 28622089 Free PMC article.
References
-
- Dourte LM, Kuntz AF, Soslowsky LJ. Twenty-five years of tendon and ligament research. J Orthop Res. 2008;26:1297–305. - PubMed
-
- Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80:4–10. - PubMed
-
- Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF, et al. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res. 2006;24:2124–32. - PubMed
-
- Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

