Seizure-induced oxidative stress in temporal lobe epilepsy
- PMID: 25650148
- PMCID: PMC4306378
- DOI: 10.1155/2015/745613
Seizure-induced oxidative stress in temporal lobe epilepsy
Abstract
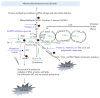
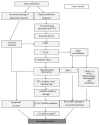
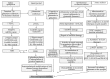
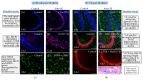
An insult to the brain (such as the first seizure) causes excitotoxicity, neuroinflammation, and production of reactive oxygen/nitrogen species (ROS/RNS). ROS and RNS produced during status epilepticus (SE) overwhelm the mitochondrial natural antioxidant defense mechanism. This leads to mitochondrial dysfunction and damage to the mitochondrial DNA. This in turn affects synthesis of various enzyme complexes that are involved in electron transport chain. Resultant effects that occur during epileptogenesis include lipid peroxidation, reactive gliosis, hippocampal neurodegeneration, reorganization of neural networks, and hypersynchronicity. These factors predispose the brain to spontaneous recurrent seizures (SRS), which ultimately establish into temporal lobe epilepsy (TLE). This review discusses some of these issues. Though antiepileptic drugs (AEDs) are beneficial to control/suppress seizures, their long term usage has been shown to increase ROS/RNS in animal models and human patients. In established TLE, ROS/RNS are shown to be harmful as they can increase the susceptibility to SRS. Further, in this paper, we review briefly the data from animal models and human TLE patients on the adverse effects of antiepileptic medications and the plausible ameliorating effects of antioxidants as an adjunct therapy.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

