α-2,3-sialyltransferase expression level impacts the kinetics of lipooligosaccharide sialylation, complement resistance, and the ability of Neisseria gonorrhoeae to colonize the murine genital tract
- PMID: 25650401
- PMCID: PMC4324315
- DOI: 10.1128/mBio.02465-14
α-2,3-sialyltransferase expression level impacts the kinetics of lipooligosaccharide sialylation, complement resistance, and the ability of Neisseria gonorrhoeae to colonize the murine genital tract
Abstract
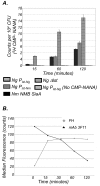
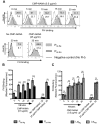
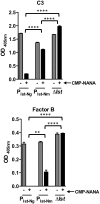
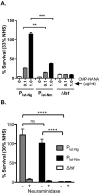
Neisseria meningitidis and Neisseria gonorrhoeae modify the terminal lacto-N-neotetraose moiety of their lipooligosaccharide (LOS) with sialic acid. N. gonorrhoeae LOS sialylation blocks killing by complement, which is mediated at least in part by enhanced binding of the complement inhibitor factor H (FH). The role of LOS sialylation in resistance of N. meningitidis to serum killing is less well defined. Sialylation in each species is catalyzed by the enzyme LOS α-2,3-sialyltransferase (Lst). Previous studies have shown increased Lst activity in N. gonorrhoeae compared to N. meningitidis due to an ~5-fold increase in lst transcription. Using isogenic N. gonorrhoeae strains engineered to express gonococcal lst from either the N. gonorrhoeae or N. meningitidis lst promoter, we show that decreased expression of lst (driven by the N. meningitidis promoter) reduced LOS sialylation as determined by less incorporation of tritium-labeled cytidine monophospho-N-acetylneuraminic acid (CMP-NANA; the donor molecule for sialic acid). Diminished LOS sialylation resulted in reduced rates of FH binding and increased pathway activation compared to N. gonorrhoeae promoter-driven lst expression. The N. meningitidis lst promoter generated sufficient Lst to sialylate N. gonorrhoeae LOS in vivo, and the level of sialylation after 24 h in the mouse genital tract was sufficient to mediate resistance to human serum ex vivo. Despite demonstrable LOS sialylation in vivo, gonococci harboring the N. meningitidis lst promoter were outcompeted by those with the N. gonorrhoeae lst promoter during coinfection of the vaginal tract of estradiol-treated mice. These data highlight the importance of high lst expression levels for gonococcal pathogenesis.
Importance: Neisseria gonorrhoeae has become resistant to nearly every therapeutic antibiotic used and is listed as an "urgent threat" by the Centers for Disease Control and Prevention. Novel therapies are needed to combat drug-resistant N. gonorrhoeae. Gonococci express an α-2,3-sialyltransferase (Lst) that can scavenge sialic acid from the host and use it to modify lipooligosaccharide (LOS). Sialylation of gonococcal LOS converts serum-sensitive strains to serum resistance, decreases antibody binding, and combats killing by neutrophils and antimicrobial peptides. Mutant N. gonorrhoeae that lack Lst (cannot sialylate LOS) are attenuated in a mouse model. Lst expression levels differ among N. gonorrhoeae strains, and N. gonorrhoeae typically expresses more Lst than Neisseria meningitidis. Here we examined the significance of differential lst expression levels and determined that the level of LOS sialylation is critical to the ability of N. gonorrhoeae to combat the immune system and survive in an animal model. LOS sialylation may be an ideal target for novel therapies.
Copyright © 2015 Lewis et al.
Figures
Similar articles
-
The Lst Sialyltransferase of Neisseria gonorrhoeae Can Transfer Keto-Deoxyoctanoate as the Terminal Sugar of Lipooligosaccharide: a Glyco-Achilles Heel That Provides a New Strategy for Vaccines to Prevent Gonorrhea.mBio. 2021 Mar 23;12(2):e03666-20. doi: 10.1128/mBio.03666-20. mBio. 2021. PMID: 33758087 Free PMC article.
-
Neisseria gonorrhoeae scavenges host sialic acid for Siglec-mediated, complement-independent suppression of neutrophil activation.mBio. 2024 May 8;15(5):e0011924. doi: 10.1128/mbio.00119-24. Epub 2024 Apr 9. mBio. 2024. PMID: 38587424 Free PMC article.
-
Therapeutic CMP-Nonulosonates against Multidrug-Resistant Neisseria gonorrhoeae.J Immunol. 2020 Jun 15;204(12):3283-3295. doi: 10.4049/jimmunol.1901398. Epub 2020 May 20. J Immunol. 2020. PMID: 32434942 Free PMC article.
-
Utilizing complement evasion strategies to design complement-based antibacterial immunotherapeutics: Lessons from the pathogenic Neisseriae.Immunobiology. 2016 Oct;221(10):1110-23. doi: 10.1016/j.imbio.2016.05.016. Epub 2016 Jun 1. Immunobiology. 2016. PMID: 27297292 Free PMC article. Review.
-
The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis.Mol Immunol. 1999 Sep-Oct;36(13-14):915-28. doi: 10.1016/s0161-5890(99)00114-5. Mol Immunol. 1999. PMID: 10698346 Review.
Cited by
-
An optimized Factor H-Fc fusion protein against multidrug-resistant Neisseria gonorrhoeae.Front Immunol. 2022 Aug 30;13:975676. doi: 10.3389/fimmu.2022.975676. eCollection 2022. Front Immunol. 2022. PMID: 36110842 Free PMC article.
-
Biological functions of sialic acid as a component of bacterial endotoxin.Front Microbiol. 2022 Oct 20;13:1028796. doi: 10.3389/fmicb.2022.1028796. eCollection 2022. Front Microbiol. 2022. PMID: 36338080 Free PMC article. Review.
-
How bacteria utilize sialic acid during interactions with the host: snip, snatch, dispatch, match and attach.Microbiology (Reading). 2022 Mar;168(3):001157. doi: 10.1099/mic.0.001157. Microbiology (Reading). 2022. PMID: 35316172 Free PMC article. Review.
-
Interferon-epsilon, an estrogen-induced type I interferon, is uniquely exploited by Neisseria gonorrhoeae via effects on sialic acid metabolism.Cell Host Microbe. 2025 Jul 9;33(7):1133-1145.e4. doi: 10.1016/j.chom.2025.05.015. Epub 2025 Jun 9. Cell Host Microbe. 2025. PMID: 40494348
-
Haemophilus parasuis α-2,3-sialyltransferase-mediated lipooligosaccharide sialylation contributes to bacterial pathogenicity.Virulence. 2018;9(1):1247-1262. doi: 10.1080/21505594.2018.1502606. Virulence. 2018. PMID: 30036124 Free PMC article.
References
-
- Joiner KA, Warren KA, Brown EJ, Swanson J, Frank MM. 1983. Studies on the mechanism of bacterial resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae. J Immunol 131:1443–1451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

