Quantitative proteomics analysis of CaMKII phosphorylation and the CaMKII interactome in the mouse forebrain
- PMID: 25650780
- PMCID: PMC4609176
- DOI: 10.1021/cn500337u
Quantitative proteomics analysis of CaMKII phosphorylation and the CaMKII interactome in the mouse forebrain
Abstract
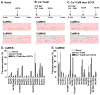
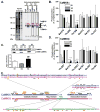
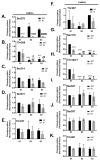
Ca(2+)/calmodulin-dependent protein kinase IIα (CaMKIIα) autophosphorylation at Thr286 and Thr305/Thr306 regulates kinase activity and modulates subcellular targeting and is critical for normal synaptic plasticity and learning and memory. Here, a mass spectrometry-based approach was used to identify Ca(2+)-dependent and -independent in vitro autophosphorylation sites in recombinant CaMKIIα and CaMKIIβ. CaMKII holoenzymes were then immunoprecipitated from subcellular fractions of forebrains isolated from either wild-type (WT) mice or mice with a Thr286 to Ala knock-in mutation of CaMKIIα (T286A-KI mice) and analyzed using the same approach in order to characterize in vivo phosphorylation sites in both CaMKII isoforms and identify CaMKII-associated proteins (CaMKAPs). A total of six and seven autophosphorylation sites in CaMKIIα and CaMKIIβ, respectively, were detected in WT mice. Thr286-phosphorylated CaMKIIα and Thr287-phosphorylated CaMKIIβ were selectively enriched in WT Triton-insoluble (synaptic) fractions compared to Triton-soluble (membrane) and cytosolic fractions. In contrast, Thr306-phosphorylated CaMKIIα and Ser315- and Thr320/Thr321-phosphorylated CaMKIIβ were selectively enriched in WT cytosolic fractions. The T286A-KI mutation significantly reduced levels of phosphorylation of CaMKIIα at Ser275 across all subcellular fractions and of cytosolic CaMKIIβ at Ser315 and Thr320/Thr321. Significantly more CaMKAPs coprecipitated with WT CaMKII holoenzymes in the synaptic fraction compared to that in the membrane fraction, with functions including scaffolding, microtubule organization, actin organization, ribosomal function, vesicle trafficking, and others. The T286A-KI mutation altered the interactions of multiple CaMKAPs with CaMKII, including several proteins linked to autism spectrum disorders. These data identify CaMKII isoform phosphorylation sites and a network of synaptic protein interactions that are sensitive to the abrogation of Thr286 autophosphorylation of CaMKIIα, likely contributing to the diverse synaptic and behavioral deficits of T286A-KI mice.
Keywords: Synaptic plasticity; autophosphorylation; mass spectrometry; postsynaptic density; protein−protein interactions; proteomics; subcellular fractionation.
Figures
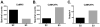


References
-
- van Woerden GM, Hoebeek FE, Gao Z, Nagaraja RY, Hoogenraad CC, Kushner SA, Hansel C, De Zeeuw CI, Elgersma Y. betaCaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nat Neurosci. 2009;12:823–825. - PubMed
-
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. - PubMed
-
- Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578. - PubMed
-
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

