Single-electron transmetalation: an enabling technology for secondary alkylboron cross-coupling
- PMID: 25650892
- PMCID: PMC4466116
- DOI: 10.1021/ja512946e
Single-electron transmetalation: an enabling technology for secondary alkylboron cross-coupling
Abstract
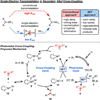
Single-electron-mediated alkyl transfer affords a novel mechanism for transmetalation, enabling cross-coupling under mild conditions. Here, general conditions are reported for cross-coupling of secondary alkyltrifluoroborates with an array of aryl bromides mediated by an Ir photoredox catalyst and a Ni cross-coupling catalyst.
Figures
References
-
- Roughley SD, Jordan AM. J. Med. Chem. 2011;54:3451. - PubMed
-
- Lozada J, Liu Z, Perrin DM. J. Org. Chem. 2014;79:5365. - PubMed
-
- Littke AF, Dai C, Fu GC. J. Am. Chem. Soc. 2000;122:4020.
- Dreher SD, Dormer PG, Sandrock DL, Molander GA. J. Am. Chem. Soc. 2008;130:9257. - PMC - PubMed
- van den Hoogenband A, Lange JHM, Terpstra JW, Koch M, Visser GM, Visser M, Korstanje TJ, Jastrzebski JTBH. Tetrahedron Lett. 2008;49:4122.
- Li L, Zhao S, Joshi-Pangu A, Diane M, Biscoe MR. J. Am. Chem. Soc. 2014;136:14027. - PMC - PubMed
-
- Hartwig JF. Organotransition Metal Chemistry: From Bonding to Catalysis. Sausalito, CA: University Science Books; 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases