Muscle as a "mediator" of systemic metabolism
- PMID: 25651178
- PMCID: PMC4398026
- DOI: 10.1016/j.cmet.2014.12.021
Muscle as a "mediator" of systemic metabolism
Abstract
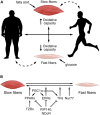
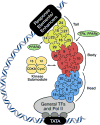
Skeletal and cardiac muscles play key roles in the regulation of systemic energy homeostasis and display remarkable plasticity in their metabolic responses to caloric availability and physical activity. In this Perspective we discuss recent studies highlighting transcriptional mechanisms that govern systemic metabolism by striated muscles. We focus on the participation of the Mediator complex in this process, and suggest that tissue-specific regulation of Mediator subunits impacts metabolic homeostasis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguère V, Evans RM. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24. - PubMed
-
- Amin RH, Mathews ST, Camp HS, Ding L, Leff T. Selective activation of PPARgamma in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2010;298:E28–E37. - PubMed
-
- Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

