Suppression of insulin production and secretion by a decretin hormone
- PMID: 25651184
- PMCID: PMC4349554
- DOI: 10.1016/j.cmet.2015.01.006
Suppression of insulin production and secretion by a decretin hormone
Erratum in
-
Suppression of Insulin Production and Secretion by a Decretin Hormone.Cell Metab. 2018 Feb 6;27(2):479. doi: 10.1016/j.cmet.2018.01.003. Cell Metab. 2018. PMID: 29414689 Free PMC article. No abstract available.
Abstract
Decretins, hormones induced by fasting that suppress insulin production and secretion, have been postulated from classical human metabolic studies. From genetic screens, we identified Drosophila Limostatin (Lst), a peptide hormone that suppresses insulin secretion. Lst is induced by nutrient restriction in gut-associated endocrine cells. limostatin deficiency led to hyperinsulinemia, hypoglycemia, and excess adiposity. A conserved 15-residue polypeptide encoded by limostatin suppressed secretion by insulin-producing cells. Targeted knockdown of CG9918, a Drosophila ortholog of Neuromedin U receptors (NMURs), in insulin-producing cells phenocopied limostatin deficiency and attenuated insulin suppression by purified Lst, suggesting CG9918 encodes an Lst receptor. NMUR1 is expressed in islet β cells, and purified NMU suppresses insulin secretion from human islets. A human mutant NMU variant that co-segregates with familial early-onset obesity and hyperinsulinemia fails to suppress insulin secretion. We propose Lst as an index member of an ancient hormone class called decretins, which suppress insulin output.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





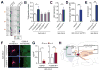
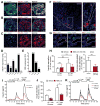
References
-
- La Barre J. Sur les possibilites d’un traitement du diabète par l’incrétine. Bull Acad R Med Belg. 1932;12:620–634.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

