A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient
- PMID: 25651363
- PMCID: PMC4526769
- DOI: 10.1038/ki.2014.382
A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient
Abstract
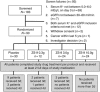
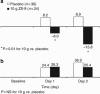
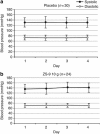
Hyperkalemia contributes to significant mortality and limits the use of cardioprotective and renoprotective renin-angiotensin-aldosterone blockers. Current therapies are poorly tolerated and not always effective. Here we conducted a phase 2 randomized, double-blind, placebo-controlled dose-escalation study to assess safety and efficacy of ZS-9. This oral selective cation exchanger that preferentially entraps potassium in the gastrointestinal tract was given to patients with stable Stage 3 chronic kidney disease and hyperkalemia (5.0 to 6.0 mEq/l) during a 2-day period. Of 90 eligible patients with mean baseline serum potassium of 5.1 mEq/l, 30 were randomized to placebo, 12-0.3 g, 24-3 g, or 24 to 10 g of ZS-9 three times daily for 2 days with regular meals. None withdrew and ZS-9 dose-dependently reduced serum potassium. The primary efficacy end point (rate of serum potassium decline in the first 48 h) was met with significance in the 3- and 10-g cohorts. From baseline, mean serum potassium was significantly decreased by 0.92±0.52 mEq/l at 38 h. Urinary potassium excretion significantly decreased with 10-g ZS-9 as compared to placebo at day 2 (+15.8 +/- 21.8 vs. +8.9 +/- 22.9 mEq per 24h) from placebo at day 2. In this short-term study, no serious adverse events were reported; only mild constipation in the 3-g dose group was possibly related to treatment. Thus, ZS-9 was well-tolerated in patients with stable chronic kidney disease and hyperkalemia leading to a rapid, sustained reduction in serum potassium.
Figures



References
-
- Jain N, Kotla S, Little BB, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–1513. - PubMed
-
- McMahon GM, Mendu ML, Gibbons FK, et al. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38:1834–1842. - PubMed
-
- Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

