Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies
- PMID: 25651368
- PMCID: PMC4424817
- DOI: 10.1038/ki.2014.423
Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies
Abstract
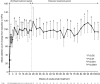
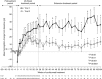
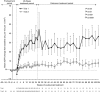
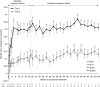
Atypical hemolytic uremic syndrome (aHUS) is a rare, possibly life-threatening disease characterized by platelet activation, hemolysis and thrombotic microangiopathy (TMA) leading to renal and other end-organ damage. We originally conducted two phase 2 studies (26 weeks and 1 year) evaluating eculizumab, a terminal complement inhibitor, in patients with progressing TMA (trial 1) and those with long duration of aHUS and chronic kidney disease (trial 2). The current analysis assessed outcomes after 2 years (median eculizumab exposure 100 and 114 weeks, respectively). At all scheduled time points, eculizumab inhibited terminal complement activity. In trial 1 with 17 patients, the platelet count was significantly improved from baseline, and hematologic normalization was achieved in 13 patients at week 26, and in 15 patients at both 1 and 2 years. The estimated glomerular filtration rate (eGFR) was significantly improved compared with baseline and year 1. In trial 2 with 20 patients, TMA event-free status was achieved by 16 patients at week 26, 17 patients at year 1, and 19 patients at year 2. Criteria for hematologic normalization were met by 18 patients at each time point. Improvement of 15 ml/min per 1.73 m(2) or more in eGFR was achieved by 1 patient at week 26, 3 patients at 1 year, and 8 patients at 2 years. The mean change in eGFR was not significant compared with baseline, week 26, or year 1. Eculizumab was well tolerated, with no new safety concerns or meningococcal infections. Thus, a 2-year analysis found that the earlier clinical benefits achieved by eculizumab treatment of aHUS were maintained at 2 years of follow-up.
Figures


Comment in
-
Managing atypical hemolytic uremic syndrome: chapter 2.Kidney Int. 2015 May;87(5):882-4. doi: 10.1038/ki.2015.60. Kidney Int. 2015. PMID: 25951068
References
-
- Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. - PubMed
-
- Zipfel PF, Heinen S, Skerka C. Thrombotic microangiopathies: new insights and new challenges. Curr Opin Nephrol Hypertens. 2010;19:372–378. - PubMed
-
- Licht C, Pluthero FG, Li L, et al. Platelet-associated complement factor H in healthy persons and patients with atypical HUS. Blood. 2009;114:4538–4545. - PubMed
-
- Azukaitis K, Loirat C, Malina M, et al. Macrovascular involvement in a child with atypical hemolytic uremic syndrome. Pediatr Nephrol. 2014;29:1273–1277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

