Endocannabinoids modulate human blood-brain barrier permeability in vitro
- PMID: 25651941
- PMCID: PMC4459020
- DOI: 10.1111/bph.13106
Endocannabinoids modulate human blood-brain barrier permeability in vitro
Abstract
Background and purpose: Endocannabinoids alter permeability at various epithelial barriers, and cannabinoid receptors and endocannabinoid levels are elevated by stroke, with potential neuroprotective effects. We therefore explored the role of endocannabinoids in modulating blood-brain barrier (BBB) permeability in normal conditions and in an ischaemia/reperfusion model.
Experimental approach: Human brain microvascular endothelial cell and astrocyte co-cultures modelled the BBB. Ischaemia was modelled by oxygen-glucose deprivation (OGD) and permeability was measured by transepithelial electrical resistance. Endocannabinoids or endocannabinoid-like compounds were assessed for their ability to modulate baseline permeability or OGD-induced hyperpermeability. Target sites of action were investigated using receptor antagonists and subsequently identified with real-time PCR.
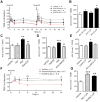
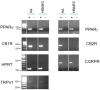
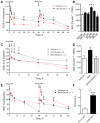
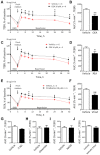
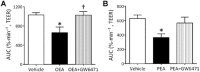
Key results: Anandamide (10 μM) and oleoylethanolamide (OEA, 10 μM) decreased BBB permeability (i.e. increased resistance). This was mediated by cannabinoid CB2 receptors, transient receptor potential vanilloid 1 (TRPV1) channels, calcitonin gene-regulated peptide (CGRP) receptor (anandamide only) and PPARα (OEA only). Application of OEA, palmitoylethanolamide (both PPARα mediated) or virodhamine (all 10 μM) decreased the OGD-induced increase in permeability during reperfusion. 2-Arachidonoyl glycerol, noladin ether and oleamide did not affect BBB permeability in normal or OGD conditions. N-arachidonoyl-dopamine increased permeability through a cytotoxic mechanism. PPARα and γ, CB1 receptors, TRPV1 channels and CGRP receptors were expressed in both cell types, but mRNA for CB2 receptors was only present in astrocytes.
Conclusion and implication: The endocannabinoids may play an important modulatory role in normal BBB physiology, and also afford protection to the BBB during ischaemic stroke, through a number of target sites.
© 2015 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures


Similar articles
-
Endocannabinoids and endocannabinoid-like compounds modulate hypoxia-induced permeability in CaCo-2 cells via CB1, TRPV1, and PPARα.Biochem Pharmacol. 2019 Oct;168:465-472. doi: 10.1016/j.bcp.2019.07.017. Epub 2019 Jul 17. Biochem Pharmacol. 2019. PMID: 31325449
-
Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors.Br J Pharmacol. 2016 Mar;173(5):815-25. doi: 10.1111/bph.13368. Epub 2016 Feb 3. Br J Pharmacol. 2016. PMID: 26497782 Free PMC article.
-
Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors.Brain Res Mol Brain Res. 2004 Dec 6;132(1):87-92. doi: 10.1016/j.molbrainres.2004.08.025. Brain Res Mol Brain Res. 2004. PMID: 15548432
-
Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain.Exp Neurol. 2010 Jul;224(1):48-55. doi: 10.1016/j.expneurol.2010.03.022. Epub 2010 Mar 29. Exp Neurol. 2010. PMID: 20353771 Review.
-
Endocannabinoids and Their Pharmacological Actions.Handb Exp Pharmacol. 2015;231:1-37. doi: 10.1007/978-3-319-20825-1_1. Handb Exp Pharmacol. 2015. PMID: 26408156 Review.
Cited by
-
Targeting the endocannabinoid system: a predictive, preventive, and personalized medicine-directed approach to the management of brain pathologies.EPMA J. 2020 Apr 15;11(2):217-250. doi: 10.1007/s13167-020-00203-4. eCollection 2020 Jun. EPMA J. 2020. PMID: 32549916 Free PMC article. Review.
-
Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation.Cardiovasc Res. 2015 Sep 1;107(4):568-78. doi: 10.1093/cvr/cvv179. Epub 2015 Jun 19. Cardiovasc Res. 2015. PMID: 26092099 Free PMC article.
-
A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells.Front Cell Neurosci. 2019 Jun 6;13:230. doi: 10.3389/fncel.2019.00230. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31244605 Free PMC article.
-
Poly (β-amino Ester) Nanoparticles Modified with a Rabies Virus-derived peptide for the Delivery of ASCL1 Across a 3D In Vitro Model of the Blood Brain Barrier.ACS Appl Nano Mater. 2023 Apr 14;6(7):6299-6311. doi: 10.1021/acsanm.3c00651. Epub 2023 Mar 31. ACS Appl Nano Mater. 2023. PMID: 37274933 Free PMC article.
-
Cannabinoids in Neurodegenerative Disorders and Stroke/Brain Trauma: From Preclinical Models to Clinical Applications.Neurotherapeutics. 2015 Oct;12(4):793-806. doi: 10.1007/s13311-015-0381-7. Neurotherapeutics. 2015. PMID: 26260390 Free PMC article. Review.
References
-
- Ahmad A, Crupi R, Impellizzeri D, Campolo M, Marino A, Esposito E, et al. Administration of palmitoylethanolamide (PEA) protects the neurovascular unit and reduces secondary injury after traumatic brain injury in mice. Brain Behav Immun. 2012a;26:1310–1321. - PubMed
-
- Ahmad A, Genovese T, Impellizzeri D, Crupi R, Velardi E, Marino A, et al. Reduction of ischemic brain injury by administration of palmitoylethanolamide after transient middle cerebral artery occlusion in rats. Brain Res. 2012b;1477:45–58. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

