IQGAP1 regulates actin cytoskeleton organization in podocytes through interaction with nephrin
- PMID: 25652011
- PMCID: PMC4356988
- DOI: 10.1016/j.cellsig.2015.01.015
IQGAP1 regulates actin cytoskeleton organization in podocytes through interaction with nephrin
Abstract
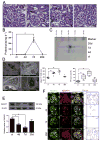
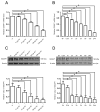
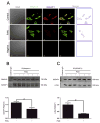
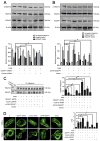
Increasing data has shown that the cytoskeletal reorganization of podocytes is involved in the onset of proteinuria and the progression of glomerular disease. Nephrin behaves as a signal sensor of the slit diaphragm to transmit cytoskeletal signals to maintain the unique structure of podocytes. However, the nephrin signaling cascade deserves further study. IQGAP1 is a scaffolding protein with the ability to regulate cytoskeletal organization. It is hypothesized that IQGAP1 contributes to actin reorganization in podocytes through interaction with nephrin. IQGAP1 expression and IQGAP1-nephrin colocalization in glomeruli were progressively decreased and then gradually recovered in line with the development of foot process fusion and proteinuria in puromycin aminonucleoside-injected rats. In cultured human podocytes, puromycin aminonucleoside-induced disruption of F-actin and disorders of migration and spreading were aggravated by IQGAP1 siRNA, and these effects were partially restored by a wild-type IQGAP1 plasmid. Furthermore, the cytoskeletal disorganization stimulated by cytochalasin D in COS7 cells was recovered by cotransfection with wild-type IQGAP1 and nephrin plasmids but was not recovered either by single transfection of the wild-type IQGAP1 plasmid or by cotransfection of mutant IQGAP1 [△1443(S→A)] and wild-type nephrin plasmids. Co-immunoprecipitation analysis using lysates of COS7 cells overexpressing nephrin and each derivative-domain molecule of IQGAP1 demonstrated that the poly-proline binding domain and RasGAP domain in the carboxyl terminus of IQGAP1 are the target modules that interact with nephrin. Collectively, these findings showed that activated IQGAP1, as an intracellular partner of nephrin, is involved in actin cytoskeleton organization and functional regulation of podocytes.
Keywords: Actin cytoskeleton; IQ domain GTPase-activating protein 1; Nephrin; Podocyte.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
IQGAP1 mediates podocyte injury in diabetic kidney disease by regulating nephrin endocytosis.Cell Signal. 2019 Jul;59:13-23. doi: 10.1016/j.cellsig.2019.03.009. Epub 2019 Mar 9. Cell Signal. 2019. PMID: 30857827
-
Nuclear translocation of IQGAP1 protein upon exposure to puromycin aminonucleoside in cultured human podocytes: ERK pathway involvement.Cell Signal. 2016 Oct;28(10):1470-8. doi: 10.1016/j.cellsig.2016.06.017. Epub 2016 Jul 1. Cell Signal. 2016. PMID: 27377965
-
Nephrin phosphorylation regulates podocyte adhesion through the PINCH-1-ILK-α-parvin complex.BMB Rep. 2013 Apr;46(4):230-5. doi: 10.5483/bmbrep.2013.46.4.270. BMB Rep. 2013. PMID: 23615266 Free PMC article.
-
Regulation of the Actin Cytoskeleton in Podocytes.Cells. 2020 Jul 16;9(7):1700. doi: 10.3390/cells9071700. Cells. 2020. PMID: 32708597 Free PMC article. Review.
-
Signaling from the podocyte intercellular junction to the actin cytoskeleton.Semin Nephrol. 2012 Jul;32(4):307-18. doi: 10.1016/j.semnephrol.2012.06.002. Semin Nephrol. 2012. PMID: 22958485 Free PMC article. Review.
Cited by
-
GAPVD1 and ANKFY1 Mutations Implicate RAB5 Regulation in Nephrotic Syndrome.J Am Soc Nephrol. 2018 Aug;29(8):2123-2138. doi: 10.1681/ASN.2017121312. Epub 2018 Jun 29. J Am Soc Nephrol. 2018. PMID: 29959197 Free PMC article.
-
Angiotensin II induces cholesterol accumulation and injury in podocytes.Sci Rep. 2017 Sep 6;7(1):10672. doi: 10.1038/s41598-017-09733-w. Sci Rep. 2017. PMID: 28878222 Free PMC article.
-
Revisiting nephrin signaling and its specialized effects on the uniquely adaptable podocyte.Biochem J. 2025 Jun 2;482(11):763-88. doi: 10.1042/BCJ20230234. Biochem J. 2025. PMID: 40457651 Free PMC article. Review.
-
Cytoskeleton Rearrangement in Podocytopathies: An Update.Int J Mol Sci. 2024 Jan 4;25(1):647. doi: 10.3390/ijms25010647. Int J Mol Sci. 2024. PMID: 38203817 Free PMC article. Review.
-
Site-specific expression of IQGAP1 in human nephrons.J Mol Histol. 2019 Apr;50(2):119-127. doi: 10.1007/s10735-019-09811-5. Epub 2019 Jan 19. J Mol Histol. 2019. PMID: 30659402
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

