Dual melanocortin-4 receptor and GLP-1 receptor agonism amplifies metabolic benefits in diet-induced obese mice
- PMID: 25652173
- PMCID: PMC4364946
- DOI: 10.15252/emmm.201404508
Dual melanocortin-4 receptor and GLP-1 receptor agonism amplifies metabolic benefits in diet-induced obese mice
Abstract
We assessed the efficacy of simultaneous agonism at the glucagon-like peptide-1 receptor (GLP-1R) and the melanocortin-4 receptor (MC4R) for the treatment of obesity and diabetes in rodents. Diet-induced obese (DIO) mice were chronically treated with either the long-acting GLP-1R agonist liraglutide, the MC4R agonist RM-493 or a combination of RM-493 and liraglutide. Co-treatment of DIO mice with RM-493 and liraglutide improves body weight loss and enhances glycemic control and cholesterol metabolism beyond what can be achieved with either mono-therapy. The superior metabolic efficacy of this combination therapy is attributed to the anorectic and glycemic actions of both drugs, along with the ability of RM-493 to increase energy expenditure. Interestingly, compared to mice treated with liraglutide alone, hypothalamic Glp-1r expression was higher in mice treated with the combination therapy after both acute and chronic treatment. Further, RM-493 enhanced hypothalamic Mc4r expression. Hence, co-dosing with MC4R and GLP-1R agonists increases expression of each receptor, indicative of minimized receptor desensitization. Together, these findings suggest potential opportunities for employing combination treatments that comprise parallel MC4R and GLP-1R agonism for the treatment of obesity and diabetes.
Keywords: Glp‐1r; Mc4r; diabetes; liraglutide; obesity.
© 2015 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

A-C Five-day treatment of DIO male mice with vehicle (white), liraglutide (10 nmol/kg) (gray), RM-493 (3.6 μmol/kg) (black), or liraglutide (10 nmol/kg) and RM-493 (3.6 μmol/kg) (checkered). Effects on (A) body weight and (B, C) body composition. Compounds were administered by daily subcutaneous injections. Data represent means ± SEM;n = 8; *P < 0.05, **P < 0.01, ***P < 0.001.
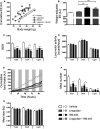
A-G Five-day treatment of DIO male mice with vehicle (white), liraglutide (10 nmol/kg) (gray), RM-493 (3.6 μmol/kg) (black), or liraglutide (10 nmol/kg) and RM-493 (3.6 μmol/kg) (checkered). Effects on (A, B) energy expenditure, (C) respiratory exchange ratio (RER), (D) locomotor activity, (E) cumulative food intake, (F) meal number and (G) meal size. Compounds were administered by daily subcutaneous injections. Data represent means ± SEM;n = 8; *P < 0.05, **P < 0.01, ***P < 0.001.
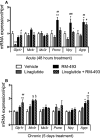
A, B Treatment-induced changes in hypothalamic gene expression in DIO mice treated for 2 (A) or 5 (B) days with vehicle (white), liraglutide (10 nmol/kg) (gray), RM-493 (3.6 μmol/kg) (black), or liraglutide (10 nmol/kg) and RM-493 (3.6 μmol/kg) (checkered). Compounds were administered by daily subcutaneous injections, and the last injection was provided 2 h prior to tissues sampling. Data represent means ± SEM;n = 8; *P < 0.05, **P < 0.01, §P < 0,001, comparison between treatment and vehicle control. #P < 0.05, ##P < 0.01, ###P < 0.001, comparison to liraglutide. +P < 0.05, comparison to RM-493.

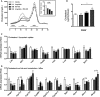
A-I Glucose metabolic parameters were assessed following 5 days of treatment of DIO male mice with vehicle (white), liraglutide (10 nmol/kg) (gray), RM-493 (3.6 μmol/kg) (black), or liraglutide (10 nmol/kg) and RM-493 (3.6 μmol/kg) (checkered). (A) Fasted blood glucose levels, (B, C) glucose tolerance, (D) fasted insulin levels, (E, F) glucose-induced insulin secretion, (G) HOMA-IR, (H) insulin sensitivity and (I) hepatic phosphorylation of AKT (p-AKTSer473) were analyzed. Compounds were administered by daily subcutaneous injections. To assess insulin-stimulated p-AKTSer473, insulin (n = 5) or saline (n = 3) was injected 10 min prior to liver sampling. Data represent means ± SEM;n = 8 in (A–H); *P < 0.05, **P < 0.01, ***P < 0.001.
A-D Cholesterol metabolic parameters were assessed following 5 days of treatment of DIO male mice with vehicle (white), liraglutide (10 nmol/kg) (gray), RM-493 (3.6 μmol/kg) (black), or liraglutide (10 nmol/kg) and RM-493 (3.6 μmol/kg) (checkered). (A) Plasma lipoprotein fractions, (B) liver cholesterol levels, (C) expression of genes implicated in hepatic cholesterol and lipoprotein uptake and (D) expression of genes implicated in cholesterol and bile acid metabolism and excretion were analyzed. Data represent means ± SEM;n = 8; *P < 0.05, **P < 0.01, ***P < 0.001.
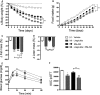
A-F 22 days of treatment of DIO male mice with vehicle (white), liraglutide (10 nmol/kg) (gray), RM-493 (3.6 μmol/kg) (black), or liraglutide (10 nmol/kg) and RM-493 (3.6 μmol/kg) (checkered). Effects on (A) body weight, (B) cumulative food intake, (C, D) body composition and (E, F) glucose tolerance. Compounds were administered by daily subcutaneous injections. Data represent means ± SEM;n = 8; *P < 0.05, **P < 0.01, ***P < 0.001.
References
-
- Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME Group NNS. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. - PubMed
-
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. - PubMed
-
- Bluher S, Ziotopoulou M, Bullen JW, Jr, Moschos SJ, Ungsunan L, Kokkotou E, Maratos-Flier E, Mantzoros CS. Responsiveness to peripherally administered melanocortins in lean and obese mice. Diabetes. 2004;53:82–90. - PubMed
-
- Castro MG, Morrison E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol. 1997;11:35–57. - PubMed
-
- Cegla J, Troke RC, Jones B, Tharakan G, Kenkre J, McCullough KA, Lim CT, Parvizi N, Hussein M, Chambers ES, et al. Co-infusion of low-dose glucagon-like peptide-1 (GLP-1) and glucagon in man results in a reduction in food intake. Diabetes. 2014;63:3711–3720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

