TGFβ signaling promotes matrix assembly during mechanosensitive embryonic salivary gland restoration
- PMID: 25652203
- PMCID: PMC4899049
- DOI: 10.1016/j.matbio.2015.01.020
TGFβ signaling promotes matrix assembly during mechanosensitive embryonic salivary gland restoration
Abstract
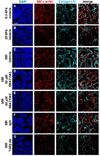
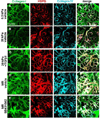
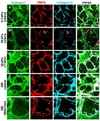
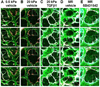
Mechanical properties of the microenvironment regulate cell morphology and differentiation within complex organs. However, methods to restore morphogenesis and differentiation in organs in which compliance is suboptimal are poorly understood. We used mechanosensitive mouse salivary gland organ explants grown at different compliance levels together with deoxycholate extraction and immunocytochemistry of the intact, assembled matrices to examine the compliance-dependent assembly and distribution of the extracellular matrix and basement membrane in explants grown at permissive or non-permissive compliance. Extracellular matrix and basement membrane assembly were disrupted in the glands grown at low compliance compared to those grown at high compliance, correlating with defective morphogenesis and decreased myoepithelial cell differentiation. Extracellular matrix and basement membrane assembly as well as myoepithelial differentiation were restored by addition of TGFβ1 and by mechanical rescue, and mechanical rescue was prevented by inhibition of TGFβ signaling during the rescue. We detected a basal accumulation of active integrin β1 in the differentiating myoepithelial cells that formed a continuous peripheral localization around the proacini and in clefts within active sites of morphogenesis in explants that were grown at high compliance. The pattern and levels of integrin β1 activation together with myoepithelial differentiation were interrupted in explants grown at low compliance but were restored upon mechanical rescue or with application of exogenous TGFβ1. These data suggest that therapeutic application of TGFβ1 to tissues disrupted by mechanical signaling should be examined as a method to promote organ remodeling and regeneration.
Keywords: Basement membrane; Branching morphogenesis; Compliance; Extracellular matrix; Integrin beta 1; Organogenesis; Salivary gland; Transforming growth factor beta.
Copyright © 2015. Published by Elsevier B.V.
Figures
Similar articles
-
Biocompatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation.Tissue Eng Part A. 2014 Jun;20(11-12):1632-42. doi: 10.1089/ten.TEA.2013.0515. Epub 2014 Feb 27. Tissue Eng Part A. 2014. PMID: 24410370 Free PMC article.
-
Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development.Organogenesis. 2016 Oct;12(4):194-216. doi: 10.1080/15476278.2016.1252887. Epub 2016 Nov 14. Organogenesis. 2016. PMID: 27841695 Free PMC article.
-
Self-organization and branching morphogenesis of primary salivary epithelial cells.Tissue Eng. 2007 Apr;13(4):721-35. doi: 10.1089/ten.2006.0123. Tissue Eng. 2007. PMID: 17341161
-
Epithelial branching morphogenesis of salivary gland: exploration of new functional regulators.J Med Invest. 2009;56 Suppl:234-8. doi: 10.2152/jmi.56.234. J Med Invest. 2009. PMID: 20224187 Review.
-
Salivary gland developmental mechanics.Curr Top Dev Biol. 2024;160:1-30. doi: 10.1016/bs.ctdb.2024.05.002. Epub 2024 May 31. Curr Top Dev Biol. 2024. PMID: 38937029 Review.
Cited by
-
Endothelial cell regulation of salivary gland epithelial patterning.Development. 2017 Jan 15;144(2):211-220. doi: 10.1242/dev.142497. Development. 2017. PMID: 28096213 Free PMC article.
-
Quantification of Confocal Images Using LabVIEW for Tissue Engineering Applications.Tissue Eng Part C Methods. 2016 Nov;22(11):1028-1037. doi: 10.1089/ten.TEC.2016.0228. Tissue Eng Part C Methods. 2016. PMID: 27758134 Free PMC article.
-
Mechanochemical Coupling and Junctional Forces during Collective Cell Migration.Biophys J. 2019 Jul 9;117(1):170-183. doi: 10.1016/j.bpj.2019.05.020. Epub 2019 May 28. Biophys J. 2019. PMID: 31200935 Free PMC article.
-
Cell type-specific transforming growth factor-β (TGF-β) signaling in the regulation of salivary gland fibrosis and regeneration.J Oral Biol Craniofac Res. 2024 May-Jun;14(3):257-272. doi: 10.1016/j.jobcr.2024.03.005. Epub 2024 Mar 21. J Oral Biol Craniofac Res. 2024. PMID: 38559587 Free PMC article.
-
Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics.Acta Biomater. 2017 Mar 1;50:437-449. doi: 10.1016/j.actbio.2016.12.049. Epub 2016 Dec 27. Acta Biomater. 2017. PMID: 28039063 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

