Increased sialidase activity in serum of cancer patients: Identification of sialidase and inhibitor activities in human serum
- PMID: 25652216
- PMCID: PMC4409881
- DOI: 10.1111/cas.12627
Increased sialidase activity in serum of cancer patients: Identification of sialidase and inhibitor activities in human serum
Abstract
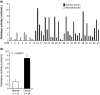
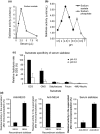
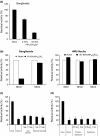
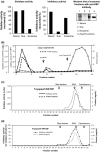
Aberrant sialylation in glycoproteins and glycolipids is a characteristic feature of malignancy. Human sialidases, which catalyze the removal of sialic acid residues from glycoconjugates, have been implicated in cancer progression. They have been detected in a wide variety of human cells and tissues, but few studies have focused on their existence in human serum. Among the four types identified to date, we previously demonstrated that plasma membrane-associated ganglioside sialidase (NEU3) is markedly upregulated in various human cancers, including examples in the colon and prostate. Here, using a sensitive assay method, we found a significant increase of sialidase activity in the serum of patients with prostate cancer compared with that in healthy subjects having low activity, if any. Activity was apparent with gangliosides as substrates, but only to a very limited extent with 4-methylumbelliferyl sialic acid, a good synthetic substrate for sialidases other than human NEU3. The serum sialidase was also almost entirely immunoprecipitated with anti-NEU3 antibody, but not with antibodies for other sialidases. Interestingly, sera additionally contained inhibitory activity against the sialidase and also against recombinant human NEU3. The sialidase and inhibitor activities could be separated by exosome isolation and by hydrophobic column chromatography. The serum sialidase was assessed by a sandwich ELISA method using two anti-NEU3 antibodies. The results provide strong evidence that the serum sialidase is, in fact, NEU3, and this subtype may, therefore, be a potential utility for novel diagnosis of human cancers.
Keywords: Cancer serum; diagnostic marker; ganglioside; prostate cancer; sialidase.
© 2015 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures

Similar articles
-
Sialidase NEU3 and its pathological significance.Glycoconj J. 2022 Oct;39(5):677-683. doi: 10.1007/s10719-022-10067-7. Epub 2022 Jun 8. Glycoconj J. 2022. PMID: 35675020 Review.
-
Roles of plasma membrane-associated sialidase NEU3 in human cancers.Biochim Biophys Acta. 2008 Mar;1780(3):532-7. doi: 10.1016/j.bbagen.2007.09.016. Epub 2007 Oct 2. Biochim Biophys Acta. 2008. PMID: 18023981
-
NEU1 and NEU3 sialidase activity expressed in human lung microvascular endothelia: NEU1 restrains endothelial cell migration, whereas NEU3 does not.J Biol Chem. 2012 May 4;287(19):15966-80. doi: 10.1074/jbc.M112.346817. Epub 2012 Mar 8. J Biol Chem. 2012. PMID: 22403397 Free PMC article.
-
Sialidase NEU3 contributes neoplastic potential on colon cancer cells as a key modulator of gangliosides by regulating Wnt signaling.Int J Cancer. 2015 Oct 1;137(7):1560-73. doi: 10.1002/ijc.29527. Epub 2015 Apr 1. Int J Cancer. 2015. PMID: 25810027
-
Inhibitors of the Sialidase NEU3 as Potential Therapeutics for Fibrosis.Int J Mol Sci. 2022 Dec 23;24(1):239. doi: 10.3390/ijms24010239. Int J Mol Sci. 2022. PMID: 36613682 Free PMC article. Review.
Cited by
-
Serum Sialylation Changes in Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Patients.J Pers Med. 2021 Oct 15;11(10):1027. doi: 10.3390/jpm11101027. J Pers Med. 2021. PMID: 34683168 Free PMC article.
-
Serum sialylation changes in cancer.Glycoconj J. 2018 Apr;35(2):139-160. doi: 10.1007/s10719-018-9820-0. Epub 2018 Apr 21. Glycoconj J. 2018. PMID: 29680984 Free PMC article. Review.
-
Exosomal Liquid Biopsy in Prostate Cancer: A Systematic Review of Biomarkers for Diagnosis, Prognosis, and Treatment Response.Int J Mol Sci. 2025 Jan 18;26(2):802. doi: 10.3390/ijms26020802. Int J Mol Sci. 2025. PMID: 39859516 Free PMC article.
-
alpha2,3 sialic acid processing enzymes expression in gastric cancer tissues reveals that ST3Gal3 but not Neu3 are associated with Lauren's classification, angiolymphatic invasion and histological grade.Eur J Histochem. 2022 Sep 29;66(4):3330. doi: 10.4081/ejh.2022.3330. Eur J Histochem. 2022. PMID: 36172711 Free PMC article.
-
Neu3 Sialidase Activates the RISK Cardioprotective Signaling Pathway during Ischemia and Reperfusion Injury (IRI).Int J Mol Sci. 2022 May 29;23(11):6090. doi: 10.3390/ijms23116090. Int J Mol Sci. 2022. PMID: 35682772 Free PMC article.
References
-
- Lau KS, Dennis JW. N-Glycans in cancer progression. Glycobiology. 2008;18:750–60. - PubMed
-
- Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22:880–96. - PubMed
-
- Miyagi T, Takahashi K, Hata K, Shiozaki K, Yamaguchi K. Sialidase significance for cancer progression. Glycoconj J. 2012;29:567–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

