Denatonium inhibits growth and induces apoptosis of airway epithelial cells through mitochondrial signaling pathways
- PMID: 25652218
- PMCID: PMC4326484
- DOI: 10.1186/s12931-015-0183-9
Denatonium inhibits growth and induces apoptosis of airway epithelial cells through mitochondrial signaling pathways
Abstract
Background: Denatonium, a widely used bitter agonist, activates bitter taste receptors on many cell types and plays important roles in chemical release, ciliary beating and smooth muscle relaxation through intracellular Ca(2+)-dependent pathways. However, the effects of denatonium on the proliferation of airway epithelial cells and on the integrity of cellular components such as mitochondria have not been studied. In this study, we hypothesize that denatonium might induce airway epithelial cell injury by damaging mitochondria.
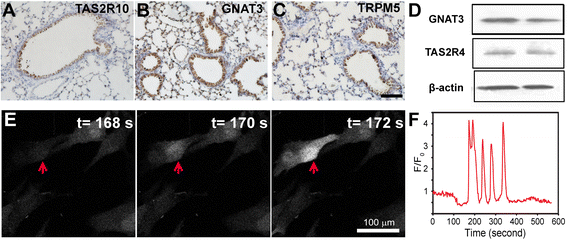
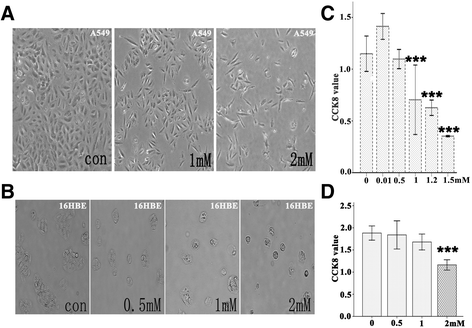
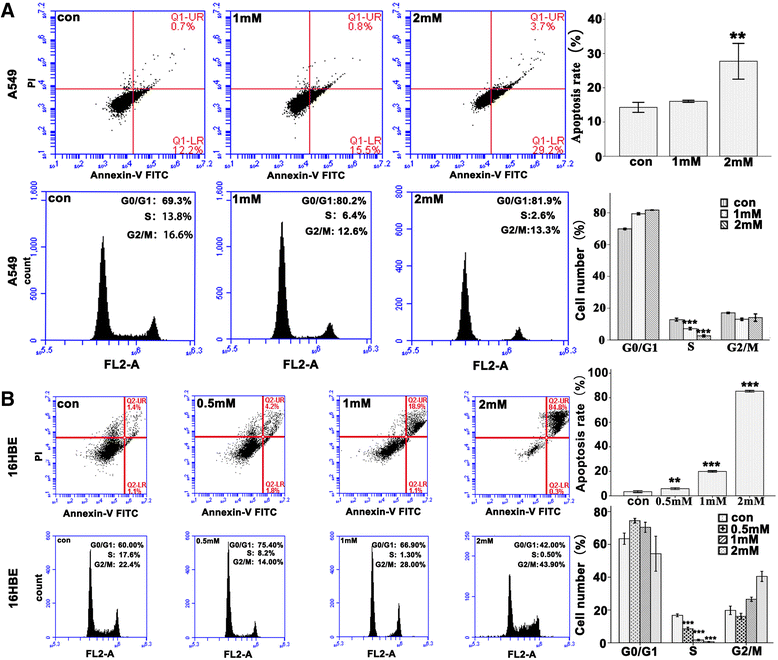
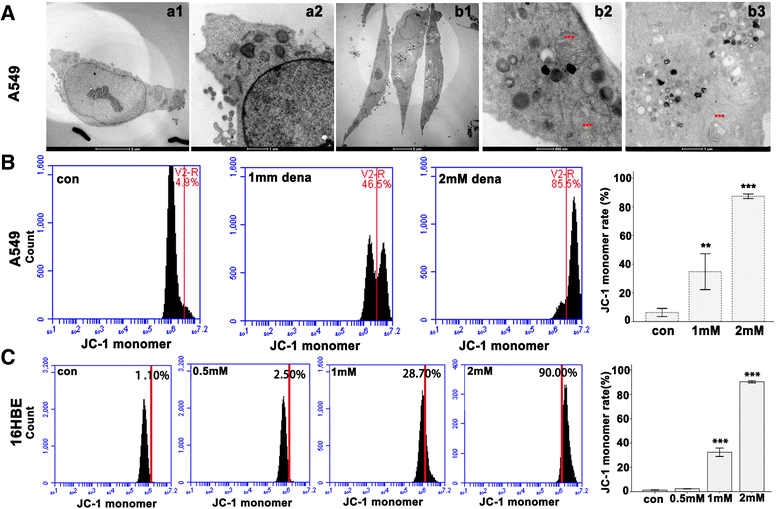
Methods: Bright-field microscopy, cell counting kit-8 (CCK-8) assay and flow cytometry analysis were used to examine cellular morphology, proliferation and cell cycle, respectively. Transmission electron microscopy (TEM) was used to examine mitochondrial integrity. JC-1 dye and western blotting techniques were used to measure mitochondrial membrane potential and protein expression, respectively.
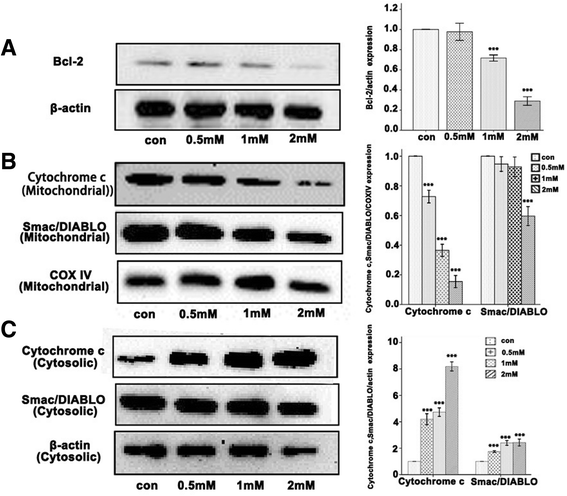
Results: For airway epithelial cells, we observed that denatonium significantly effects cellular morphology, decreases cell proliferation and reduces the number of cells in S phase in a dose-dependent manner. TEM analysis demonstrated that denatonium causes large amplitude swelling of mitochondria, which was confirmed by the loss of mitochondrial membrane potential, the down-regulation of Bcl-2 protein and the subsequent enhancement of the mitochondrial release of cytochrome c and Smac/DIABLO after denatonium treatment.
Conclusions: In this study, we demonstrated for the first time that denatonium damages mitochondria and thus induces apoptosis in airway epithelial cells.
Figures
References
-
- Ogura T, Margolskee RF, Kinnamon SC. Taste receptor cell responses to the bitter stimulus denatonium involve Ca2+ influx via store-operated channels. J Neurophysiol. 2002;87:3152–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

