Maternal plasma and breastmilk viral loads are associated with HIV-1-specific cellular immune responses among HIV-1-exposed, uninfected infants in Kenya
- PMID: 25652232
- PMCID: PMC4449779
- DOI: 10.1111/cei.12599
Maternal plasma and breastmilk viral loads are associated with HIV-1-specific cellular immune responses among HIV-1-exposed, uninfected infants in Kenya
Abstract
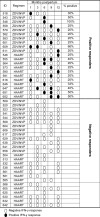
Infants exposed to maternal HIV-1 provide an opportunity to assess correlates of HIV-1-specific interferon (IFN)-γ responses and may be informative in the development of HIV-1 vaccines. HIV-1-infected women with CD4 counts 200-500 cells/mm(3) were randomized to short-course zidovudine/nevirapine (ZDV/NVP) or highly active anti-retroviral therapy (HAART) between 2003 and 2005. Maternal plasma and breastmilk HIV-1 RNA and DNA were quantified during the first 6-12 months postpartum. HIV-1 gag peptide-stimulated enzyme-linked immunospot (ELISPOT) assays were conducted in HIV-1-exposed, uninfected infants (EU), and correlates were determined using regression and generalized estimating equations. Among 47 EU infants, 21 (45%) had ≥1 positive ELISPOT result during follow-up. Infants had a median response magnitude of 177 HIV-1-specific spot-forming units (SFU)/106 peripheral blood mononuclear cells (PBMC) [interquartile range (IQR)=117-287] directed against 2 (IQR = 1-3) gag peptide pools. The prevalence and magnitude of responses did not differ by maternal anti-retroviral (ARV) randomization arm. Maternal plasma HIV-1 RNA levels during pregnancy (P=0.009) and breastmilk HIV-1 DNA levels at 1 month (P=0.02) were associated with a higher magnitude of infant HIV-1-specific ELISPOT responses at 1 month postpartum. During follow-up, concurrent breastmilk HIV-1 RNA and DNA (cell-free virus and cell-associated virus, respectively) each were associated positively with magnitude of infant HIV-1-specific responses (P=0.01). Our data demonstrate the importance of antigenic exposure on the induction of infant HIV-1-specific cellular immune responses in the absence of infection.
Keywords: HIV-1-EU; interferon gamma; paediatric immunity.
© 2015 British Society for Immunology.
Figures

References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) 2010. Global report: UNAIDS report on the global AIDS epidemic 2010;. Available at: http://www.unaids.org/globalreport/Global_report.html. - PubMed
-
- Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–74. - PubMed
-
- Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

