Analysis of hedgehog signaling in cerebellar granule cell precursors in a conditional Nsdhl allele demonstrates an essential role for cholesterol in postnatal CNS development
- PMID: 25652406
- PMCID: PMC4406293
- DOI: 10.1093/hmg/ddv042
Analysis of hedgehog signaling in cerebellar granule cell precursors in a conditional Nsdhl allele demonstrates an essential role for cholesterol in postnatal CNS development
Abstract
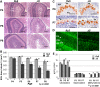
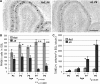
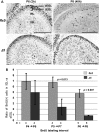
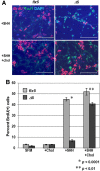
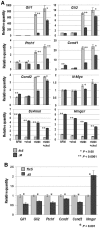
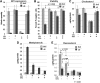
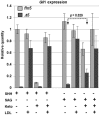
NSDHL is a 3β-hydroxysterol dehydrogenase that is involved in the removal of two C-4 methyl groups in one of the later steps of cholesterol biosynthesis. Mutations in the gene encoding the enzyme are responsible for the X-linked, male lethal mouse mutations bare patches and striated, as well as most cases of human CHILD syndrome. Rare, hypomorphic NSDHL mutations are also associated with X-linked intellectual disability in males with CK syndrome. Since hemizygous male mice with Nsdhl mutations die by midgestation, we generated a conditional targeted Nsdhl mutation (Nsdhl(tm1.1Hrm)) to investigate the essential role of cholesterol in the early postnatal CNS. Ablation of Nsdhl in radial glia using GFAP-cre resulted in live-born, normal appearing affected male pups. However, the pups develop overt ataxia by postnatal day 8-10 and die shortly thereafter. Histological abnormalities include progressive loss of cortical and hippocampal neurons, as well as deficits in the proliferation and migration of cerebellar granule precursors and subsequent massive apoptosis of the cerebellar cortex. We replicated the granule cell precursor proliferation defect in vitro and demonstrate that it results from defective signaling by SHH. Furthermore, this defect is almost completely rescued by supplementation of the culture media with exogenous cholesterol, while methylsterol accumulation above the enzymatic block appears to be associated with increased cell death. These data support the absolute requirement for cholesterol synthesis in situ once the blood-brain-barrier forms and cholesterol transport to the fetus is abolished. They further emphasize the complex ramifications of cholesterogenic enzyme deficiency on cellular metabolism.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Saher G., Quintes S., Nave K.A. (2011) Cholesterol: a novel regulatory role in myelin formation. Neuroscientist, 17, 79–93. - PubMed
-
- Herman G.E. (2003) Disorders of cholesterol biosynthesis: prototypic metabolic malformation syndromes. Hum. Mol. Genet., 12 Spec No 1, R75–R88. - PubMed
-
- Mann R.K., Beachy P.A. (2004) Novel lipid modifications of secreted protein signals. Annu. Rev. Biochem., 73, 891–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

