Independent calculation of monitor units for VMAT and SPORT
- PMID: 25652504
- PMCID: PMC4312348
- DOI: 10.1118/1.4906185
Independent calculation of monitor units for VMAT and SPORT
Abstract
Purpose: Dose and monitor units (MUs) represent two important facets of a radiation therapy treatment. In current practice, verification of a treatment plan is commonly done in dose domain, in which a phantom measurement or forward dose calculation is performed to examine the dosimetric accuracy and the MU settings of a given treatment plan. While it is desirable to verify directly the MU settings, a computational framework for obtaining the MU values from a known dose distribution has yet to be developed. This work presents a strategy to calculate independently the MUs from a given dose distribution of volumetric modulated arc therapy (VMAT) and station parameter optimized radiation therapy (SPORT).
Methods: The dose at a point can be expressed as a sum of contributions from all the station points (or control points). This relationship forms the basis of the proposed MU verification technique. To proceed, the authors first obtain the matrix elements which characterize the dosimetric contribution of the involved station points by computing the doses at a series of voxels, typically on the prescription surface of the VMAT/SPORT treatment plan, with unit MU setting for all the station points. An in-house Monte Carlo (MC) software is used for the dose matrix calculation. The MUs of the station points are then derived by minimizing the least-squares difference between doses computed by the treatment planning system (TPS) and that of the MC for the selected set of voxels on the prescription surface. The technique is applied to 16 clinical cases with a variety of energies, disease sites, and TPS dose calculation algorithms.
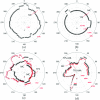
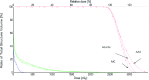
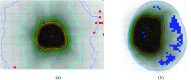
Results: For all plans except the lung cases with large tissue density inhomogeneity, the independently computed MUs agree with that of TPS to within 2.7% for all the station points. In the dose domain, no significant difference between the MC and Eclipse Anisotropic Analytical Algorithm (AAA) dose distribution is found in terms of isodose contours, dose profiles, gamma index, and dose volume histogram (DVH) for these cases. For the lung cases, the MC-calculated MUs differ significantly from that of the treatment plan computed using AAA. However, the discrepancies are reduced to within 3% when the TPS dose calculation algorithm is switched to a transport equation-based technique (Acuros™). Comparison in the dose domain between the MC and Eclipse AAA/Acuros calculation yields conclusion consistent with the MU calculation.
Conclusions: A computational framework relating the MU and dose domains has been established. The framework does not only enable them to verify the MU values of the involved station points of a VMAT plan directly in the MU domain but also provide a much needed mechanism to adaptively modify the MU values of the station points in accordance to a specific change in the dose domain.
Figures

Similar articles
-
Secondary monitor unit calculations for VMAT using parallelized Monte Carlo simulations.J Appl Clin Med Phys. 2019 Jun;20(6):60-69. doi: 10.1002/acm2.12605. Epub 2019 May 24. J Appl Clin Med Phys. 2019. PMID: 31127699 Free PMC article.
-
A Monte Carlo tool for evaluating VMAT and DIMRT treatment deliveries including planar detectors.Phys Med Biol. 2013 Jun 7;58(11):3535-50. doi: 10.1088/0031-9155/58/11/3535. Epub 2013 May 2. Phys Med Biol. 2013. PMID: 23640066
-
On the use of a convolution-superposition algorithm for plan checking in lung stereotactic body radiation therapy.J Appl Clin Med Phys. 2016 Sep 8;17(5):99-110. doi: 10.1120/jacmp.v17i5.6186. J Appl Clin Med Phys. 2016. PMID: 27685114 Free PMC article.
-
Challenges in calculation of the gamma index in radiotherapy - Towards good practice.Phys Med. 2017 Apr;36:1-11. doi: 10.1016/j.ejmp.2017.03.001. Epub 2017 Mar 14. Phys Med. 2017. PMID: 28410677 Review.
-
Impact of dose calculation algorithm on radiation therapy.World J Radiol. 2014 Nov 28;6(11):874-80. doi: 10.4329/wjr.v6.i11.874. World J Radiol. 2014. PMID: 25431642 Free PMC article. Review.
Cited by
-
Monte Carlo modeling of ultrasound probes for image guided radiotherapy.Med Phys. 2015 Oct;42(10):5745-56. doi: 10.1118/1.4929978. Med Phys. 2015. PMID: 26429248 Free PMC article.
-
Secondary monitor unit calculations for VMAT using parallelized Monte Carlo simulations.J Appl Clin Med Phys. 2019 Jun;20(6):60-69. doi: 10.1002/acm2.12605. Epub 2019 May 24. J Appl Clin Med Phys. 2019. PMID: 31127699 Free PMC article.
-
A Homogeneous Water-Equivalent Anthropomorphic Phantom for Dosimetric Verification of Radiotherapy Plans.J Med Phys. 2018 Apr-Jun;43(2):100-105. doi: 10.4103/jmp.JMP_123_17. J Med Phys. 2018. PMID: 29962687 Free PMC article.
-
Monitor unit verification for Varian TrueBeam VMAT plans using Monte Carlo calculations and phase space data.J Appl Clin Med Phys. 2023 Oct;24(10):e14063. doi: 10.1002/acm2.14063. Epub 2023 Jul 19. J Appl Clin Med Phys. 2023. PMID: 37469244 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

