Non-myeloablative allogeneic hematopoietic cell transplantation following fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: a phase II randomized study from the Belgian Hematological Society
- PMID: 25652604
- PMCID: PMC4332717
- DOI: 10.1186/s13045-014-0098-9
Non-myeloablative allogeneic hematopoietic cell transplantation following fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: a phase II randomized study from the Belgian Hematological Society
Abstract
Background: Few studies thus far have compared head-to-head different non-myelooablative conditioning regimens for allogeneic hematopoietic cell transplantation (allo-HCT).
Methods: Here, we report the results of a phase II multicenter randomized study comparing non-myeloablative allo-HCT from HLA-identical siblings (n = 54) or from 10/10 HLA-matched unrelated donors (n = 40) with either fludarabine plus 2 Gy total body irradiation (Flu-TBI arm; n = 49) or 8 Gy TLI + anti-thymocyte globulin (TLI-ATG arm; n = 45) conditioning.
Results: The 180-day cumulative incidences of grade II-IV acute GVHD (primary endpoint) were 12.2% versus 8.9% in Flu-TBI and TLI-ATG patients, respectively (P = 0.5). Two-year cumulative incidences of moderate/severe chronic GVHD were 40.8% versus 17.8% in Flu-TBI and TLI-ATG patients, respectively (P = 0.017). Five Flu-TBI patients and 10 TLI-ATG patients received pre-emptive DLI for low donor chimerism levels, while 1 Flu-TBI patient and 5 TLI-ATG patients (including 2 patients given prior pre-emptive DLIs) received a second HCT for poor graft function, graft rejection, or disease progression. Four-year cumulative incidences of relapse/progression were 22% and 50% in Flu-TBI and TLI-ATG patients, respectively (P = 0.017). Four-year cumulative incidences of nonrelapse mortality were 24% and 13% in Flu-TBI and TLI-ATG patients, respectively (P = 0.5). Finally, 4-year overall (OS) and progression-free survivals (PFS) were 53% and 54%, respectively, in the Flu-TBI arm, versus 54% (P = 0.9) and 37% (P = 0.12), respectively, in the TLI-ATG arm.
Conclusions: In comparison to patients included in the Flu-TBI arm, patients included in the TLI-ATG arm had lower incidence of chronic GVHD, higher incidence of relapse and similar OS.
Trial registration: The study was registered on ClinicalTrial.gov ( NCT00603954 ) and EUDRACT (2010-024297-19) .
Figures
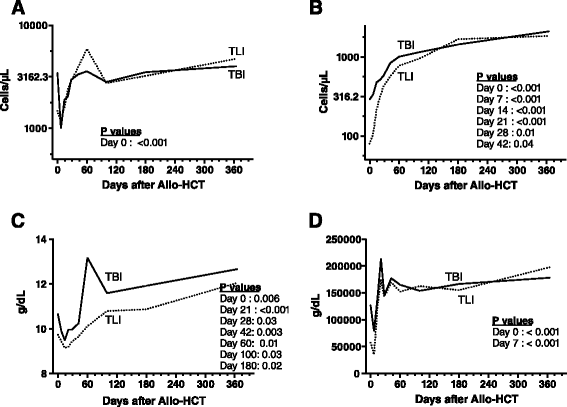
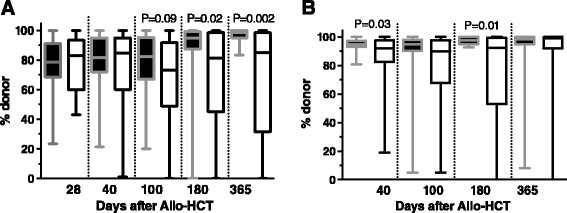
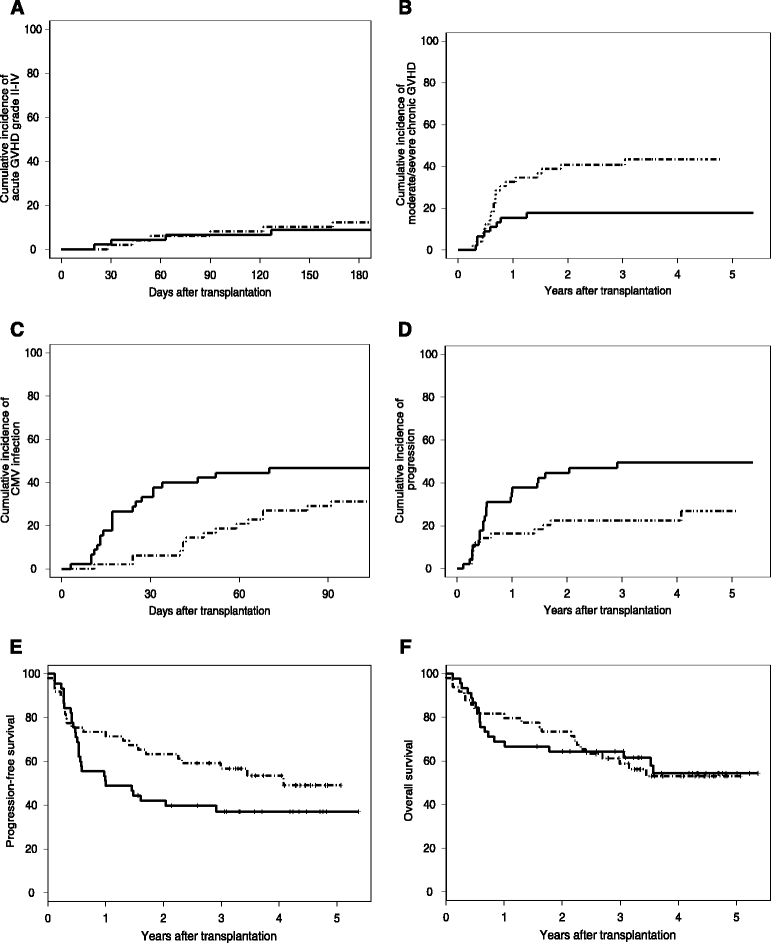
References
-
- Sorror ML, Sandmaier BM, Storer BE, Franke GN, Laport GG, Chauncey TR, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. J Am Med Assoc. 2011;306:1874–83. doi: 10.1001/jama.2011.1558. - DOI - PMC - PubMed
-
- McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. doi: 10.1182/blood.V97.11.3390. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

