Formulation of Eco-friendly Medicated Chewing Gum to Prevent Motion Sickness
- PMID: 25652732
- PMCID: PMC4674635
- DOI: 10.1208/s12249-015-0296-y
Formulation of Eco-friendly Medicated Chewing Gum to Prevent Motion Sickness
Abstract
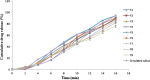
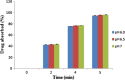
An attempt was made to formulate medicated chewing gum to prevent motion sickness using natural gum base for faster onset of action and easy administration, anywhere and anytime, without access to water. To avoid the discard issue of gum cud, natural gum base of Triticum aestivum (wheat grain) was explored because of its biodegradable and biocompatible nature and easy availability. Prolamin, extracted from wheat, showed good chewing capacity, elasticity, high water retention capacity, antifungal activity, and compatibility with the drug. Formulations were prepared based on a two-factor and three-level factorial design. Amount of calcium carbonate (texturizer) and gum base were selected as independent variables. Elasticity and drug release were considered as the dependent variables. All batches were evaluated for the content uniformity, elasticity study, texture study, in vitro drug release study, and chewiness study. Results revealed that medicated chewing gum containing 80 mg of calcium carbonate and 500 mg of gum base showed good elasticity and more than 90% drug release within 16 min. Thus, this study suggested that both good elasticity and chew ability and abundant availability of wheat grain can act as a potential gum base for medicated chewing gum.
Keywords: biodegradable gum; gum base; medicated chewing gum; plasticizer; wheat prolamin.
Figures






References
-
- Rassing MR. Chewing gum as a drug delivery system. Adv Drug Delivery Rev. 1994;13:89–121. doi: 10.1016/0169-409X(94)90028-0. - DOI
-
- General monograph on dosage forms. Chewing gums, medicated, drug release. In: European Pharmacopoeia, 4th ed.; European directorate for the quality of medicines, Council of Europe: Strasbourg. France; 2002.p. 227.
-
- Barcelon SA. Chewing gum containing wheat gluten. Patent number WO1994017673 A1, Aug 18; 1994.
-
- Sramkova Z, Gregova E, Sturdik E. Chemical composition and nutritional quality of wheat grain. Acta Chimica Slovaca. 2009;2:115–38.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

