The assembly dynamics of the cytolytic pore toxin ClyA
- PMID: 25652783
- PMCID: PMC4347018
- DOI: 10.1038/ncomms7198
The assembly dynamics of the cytolytic pore toxin ClyA
Erratum in
-
Erratum: The assembly dynamics of the cytolytic pore toxin ClyA.Nat Commun. 2016 Feb 1;7:10650. doi: 10.1038/ncomms10650. Nat Commun. 2016. PMID: 26830972 Free PMC article. No abstract available.
Abstract
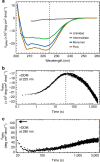
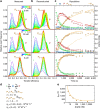
Pore-forming toxins are protein assemblies used by many organisms to disrupt the membranes of target cells. They are expressed as soluble monomers that assemble spontaneously into multimeric pores. However, owing to their complexity, the assembly processes have not been resolved in detail for any pore-forming toxin. To determine the assembly mechanism for the ring-shaped, homododecameric pore of the bacterial cytolytic toxin ClyA, we collected a diverse set of kinetic data using single-molecule spectroscopy and complementary techniques on timescales from milliseconds to hours, and from picomolar to micromolar ClyA concentrations. The entire range of experimental results can be explained quantitatively by a surprisingly simple mechanism. First, addition of the detergent n-dodecyl-β-D-maltopyranoside to the soluble monomers triggers the formation of assembly-competent toxin subunits, accompanied by the transient formation of a molten-globule-like intermediate. Then, all sterically compatible oligomers contribute to assembly, which greatly enhances the efficiency of pore formation compared with simple monomer addition.
Figures




References
-
- Iacovache I., van der Goot F. G. & Pernot L. Pore formation: an ancient yet complex form of attack. Biochim. Biophys. Acta 1778, 1611–1623 (2008). - PubMed
-
- Iacovache I., Degiacomi M. T. & van der Goot F. G. inComprehensive Biophysics ed. Egelman E. Elsevier Science (2012).
-
- Song L., Hobaugh M. R., Shustak C., Cheley S., Bayley H. & Gouaux J. E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 274, 1859–1866 (1996). - PubMed
-
- Tschopp J. Ultrastructure of the membrane attack complex of complement. Heterogeneity of the complex caused by different degree of C9 polymerization. J. Biol. Chem. 259, 7857–7863 (1984). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

