Surround suppression and temporal processing of visual signals
- PMID: 25652919
- PMCID: PMC4416570
- DOI: 10.1152/jn.00480.2014
Surround suppression and temporal processing of visual signals
Abstract
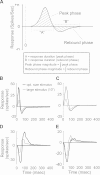
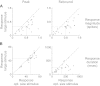
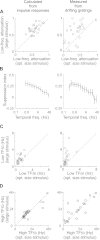
Extraclassical surround suppression strongly modulates responses of neurons in the retina, lateral geniculate nucleus (LGN), and primary visual cortex. Although a great deal is known about the spatial properties of extraclassical suppression and the role it serves in stimulus size tuning, relatively little is known about how extraclassical suppression shapes visual processing in the temporal domain. We recorded the spiking activity of retinal ganglion cells and LGN neurons in the cat to test the hypothesis that extraclassical suppression influences temporal features of visual responses in the early visual system. Our results demonstrate that extraclassical suppression not only shifts the distribution of interspike intervals in a manner that decreases the efficacy of neuronal communication, it also decreases the reliability of neuronal responses to visual stimuli and it decreases the duration of visual responses, an effect that underlies a rightward shift in the temporal frequency tuning of LGN neurons. Taken together, these results reveal a dynamic relationship between extraclassical suppression and the temporal features of neuronal responses.
Keywords: cat; extraclassical suppression; lateral geniculate nucleus; nonlinear receptive field.
Copyright © 2015 the American Physiological Society.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

