Transport domain unlocking sets the uptake rate of an aspartate transporter
- PMID: 25652997
- PMCID: PMC4351760
- DOI: 10.1038/nature14158
Transport domain unlocking sets the uptake rate of an aspartate transporter
Abstract
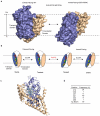
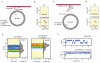
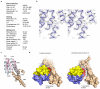
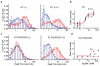
Glutamate transporters terminate neurotransmission by clearing synaptically released glutamate from the extracellular space, allowing repeated rounds of signalling and preventing glutamate-mediated excitotoxicity. Crystallographic studies of a glutamate transporter homologue from the archaeon Pyrococcus horikoshii, GltPh, showed that distinct transport domains translocate substrates into the cytoplasm by moving across the membrane within a central trimerization scaffold. Here we report direct observations of these 'elevator-like' transport domain motions in the context of reconstituted proteoliposomes and physiological ion gradients using single-molecule fluorescence resonance energy transfer (smFRET) imaging. We show that GltPh bearing two mutations introduced to impart characteristics of the human transporter exhibits markedly increased transport domain dynamics, which parallels an increased rate of substrate transport, thereby establishing a direct temporal relationship between transport domain motion and substrate uptake. Crystallographic and computational investigations corroborated these findings by revealing that the 'humanizing' mutations favour structurally 'unlocked' intermediate states in the transport cycle exhibiting increased solvent occupancy at the interface between the transport domain and the trimeric scaffold.
Figures











References
-
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–7. - PubMed
-
- Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–8. - PubMed
-
- Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–93. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 EB003150/EB/NIBIB NIH HHS/United States
- R01 GM098859/GM/NIGMS NIH HHS/United States
- 5U54GM087519/GM/NIGMS NIH HHS/United States
- R010EB003150/EB/NIBIB NIH HHS/United States
- R01GM098859/GM/NIGMS NIH HHS/United States
- U54 GM087519/GM/NIGMS NIH HHS/United States
- R21 MH099491/MH/NIMH NIH HHS/United States
- P01DA012408/DA/NIDA NIH HHS/United States
- P01 DA012408/DA/NIDA NIH HHS/United States
- R21MH099491/MH/NIMH NIH HHS/United States
- P41 GM103521/GM/NIGMS NIH HHS/United States
- P41GM103521/GM/NIGMS NIH HHS/United States
- R37 NS085318/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

